The global Pharmaceutical Regulatory Affairs market is expected to grow at a moderate rate during the forecast period. An increase in demand for the faster approval process and changes in the regulatory landscape in the healthcare sector are boosting the growth of the global healthcare biometric market. An increasing growing field including specialty therapies, orphan drugs, and personalized expands the growth of the market.
Moreover, governments in many countries are supporting and taking initiatives for the adoption of pharmaceutical regulatory affairs is the important factor expected to boost the growth of the Pharmaceutical Regulatory Affairs market in near future. In addition to this, growing health care expenditure is further driving the expansion of this market globally.
On the other hand, healthcare along with the challenges associated with the pharmaceutical regulatory affairs is likely to hamper the global Pharmaceutical Regulatory Affairs market during the forecast period.
Covid-19 Impact on Pharmaceutical Regulatory Affairs Market:
In addition, the current Pharmaceutical Regulatory Affairs Market study offers a detailed analysis of the current COVID-19 pandemic impact on the market growth and its influence on the future growth of the Pharmaceutical Regulatory Affairs Market. The recently published report demonstrates the elevation in the demand for the healthcare sector. The healthcare manufacturers have experienced long term as well as short term effect which includes supply shortages, panic buying and stocking, regulation changes as short-term whereas approval delays and possible trend variations in consumption could be perceived as long-term impacts of COVID-19 on the health and pharmaceutical market.
The increasing need for a cure has pushed vaccine research and manufacturers to the limit. In addition to this, panic conditions have already spurred the demand for many healthcare products and services which are discussed in detail in this report. Moreover, the impact of COVID-19 on overall market revenue for the base year 2020 and its projection up to 2027 is provided in detail in this report.
Pharmaceutical Regulatory Affairs Market Segment Overview
According to Product type, the market is segmented into Regulatory Consulting, Legal Representation, Regulatory Writing and Publishing, Product Registration and Clinical Trial Application, and Other Services. Among these Regulatory Consulting holds the largest share of the market mainly because of the growing incidences of novel diseases which requires newer products for combatting with the disease, propelling the demand for regulatory consulting. This segment is hence anticipated to propel the growth of the pharmaceutical regulatory affairs market in the coming years.
Based on application, the market is further segmented into in-house and outsourcing. In addition, outsourcing segment are expected to grow at the highest rate in the coming years. Furthermore, surge in innovations and development of new products for the management of the diseases, requiring fulfilment of regulatory norms and higher cost of such testing leads the companies to outsource the regulatory services, thus boosting the demand for Pharmaceutical Regulatory Affairs globally.
Market Analysis, Insights and Forecast – By Product type
· Regulatory Consulting
· Legal Representation
· Regulatory Writing and Publishing
· Product Registration and Clinical Trial Application
· Other Services
Market Analysis, Insights and Forecast – By Application
· In-house
· Outsourcing
Pharmaceutical Regulatory Affairs Market Regional Overview
Region-wise, North America dominated the global Pharmaceutical Regulatory Affairs market and is expected to continue its dominance during the forecast period. Factors such as the rising number of product innovations, and high health care expenditure propels the growth of the market in North America. Similarly, Asia Pacific is also expected to account for the fastest growth in the global market owing to well-established health care infrastructure. Factors such as development in the health care infrastructure, rising awareness about health, a surge in investment in pharmaceuticals and biotechnology sectors, and growing adoption of outsourcing services for fulfilment of regulatory norms given by authorities of a specific country, are expected to escalate the growth of the Pharmaceutical Regulatory Affairs market in the Asia Pacific region.
Pharmaceutical Regulatory Affairs Market, By Geography
· North America (US & Canada)
· Europe (UK, Germany, France, Italy, Spain, & Rest of Europe)
· Asia-Pacific (Japan, China, India, Australia, & South Korea, & Rest of Asia-Pacific)
· LAMEA (Brazil, Saudi Arabia, UAE & Rest of LAMEA)
Pharmaceutical Regulatory Affairs Market Competitor overview
Some key developments and strategies adopted by manufacturers in the Pharmaceutical Regulatory Affairs are highlighted below.
· In March 2021, PharmaVentures announced that it acted as exclusive M&A advisor on the sale of the ERA Consulting Group to PharmaLex GmbH. ERA Consulting is a leading strategic product development and regulatory consulting group serving the global biopharmaceutical industry. This would help the pharmaceutical regulatory affairs market gain traction in the coming years.
Pharmaceutical Regulatory Affairs Market, Key Players
· QuintilesIMS
· LabCor
· PAREXEL
· Pharmaceutical Product Development
· RESEARCH HOLDINGS INC
· PRA Health Sciences
· ICON
· Charles River
· Advent International
· WuXi AppTec, Inc.
· Pharmaron Beijing Co., Ltd.
· Hangzhou Tigermed Consulting Co.,Ltd.
· Asymchem Laboratories (Tianjin) Co.,Ltd.
· Accell Clinical Research, LLC.
· GenPact Ltd.
1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
2. Executive Summary
3. Market Dynamics
- 3.1. Market Drivers
- 3.2. Market Restraints
- 3.3. Market Opportunities
4. Key Insights
- 4.1. Key Emerging Trends – For Major Countries
- 4.2. Latest Technological Advancement
- 4.3. Regulatory Landscape
- 4.4. Industry SWOT Analysis
- 4.5. Porters Five Forces Analysis
5. Global Pharmaceutical Regulatory Affairs Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 5.1. Key Findings / Summary
- 5.2. Market Analysis, Insights and Forecast – By Product type
- 5.2.1. Regulatory Consulting
- 5.2.2. Legal Representation
- 5.2.3. Regulatory Writing and Publishing
- 5.2.4. Product Registration and Clinical Trial Application
- 5.2.5. Other Services
- 5.3. Market Analysis, Insights and Forecast – By Application
- 5.3.1. In-house
- 5.3.2. Outsourcing
- 5.4. Market Analysis, Insights and Forecast – By Region
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Latin America, Middle East, and Africa
6. North America Pharmaceutical Regulatory Affairs Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 6.1. Key Findings / Summary
- 6.2. Market Analysis, Insights and Forecast – By Product type
- 6.2.1. Regulatory Consulting
- 6.2.2. Legal Representation
- 6.2.3. Regulatory Writing and Publishing
- 6.2.4. Product Registration and Clinical Trial Application
- 6.2.5. Other Services
- 6.3. Market Analysis, Insights and Forecast – By Application
- 6.3.1. In-house
- 6.3.2. Outsourcing
- 6.4. Market Analysis, Insights and Forecast – By Country
- 6.4.1. U.S.
- 6.4.2. Canada
7. Europe Pharmaceutical Regulatory Affairs Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 7.1. Key Findings / Summary
- 7.2. Market Analysis, Insights and Forecast – By Product type
- 7.2.1. Regulatory Consulting
- 7.2.2. Legal Representationi
- 7.2.3. Regulatory Writing and Publishing
- 7.2.4. Product Registration and Clinical Trial Application
- 7.2.5. Other Services
- 7.3. Market Analysis, Insights and Forecast – By Application
- 7.3.1. In-house
- 7.3.2. Outsourcing
- 7.4. Market Analysis, Insights and Forecast – By Country
- 7.4.1. UK
- 7.4.2. Germany
- 7.4.3. France
- 7.4.4. Italy
- 7.4.5. Spain
- 7.4.6. Russia
- 7.4.7. Rest of Europe
8. Asia Pacific Pharmaceutical Regulatory Affairs Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 8.1. Key Findings / Summary
- 8.2. Market Analysis, Insights and Forecast – By Product type
- 8.2.1. Regulatory Consulting
- 8.2.2. Legal Representation
- 8.2.3. Regulatory Writing and Publishing
- 8.2.4. Product Registration and Clinical Trial Application
- 8.2.5. Other Services
- 8.3. Market Analysis, Insights and Forecast – By Application
- 8.3.1. In-house
- 8.3.2. Outsourcing
- 8.4. Market Analysis, Insights and Forecast – By Country
- 8.4.1. China
- 8.4.2. India
- 8.4.3. Japan
- 8.4.4. Australia
- 8.4.5. South East Asia
- 8.4.6. Rest of Asia Pacific
9. Latin America, Middle East, and Africa Pharmaceutical Regulatory Affairs Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 9.1. Key Findings / Summary
- 9.2. Market Analysis, Insights and Forecast – By Product type
- 9.2.1. Regulatory Consulting
- 9.2.2. Legal Representation
- 9.2.3. Regulatory Writing and Publishing
- 9.2.4. Product Registration and Clinical Trial Application
- 9.2.5. Other Services
- 9.3. Market Analysis, Insights and Forecast – By Application
- 9.3.1. In-house
- 9.3.2. Outsourcing
- 9.4. Market Analysis, Insights and Forecast – By Country
- 9.4.1. Brazil
- 9.4.2. Saudi Arabia
- 9.4.3. UAE
- 9.4.4. Rest of LAMEA
10. Competitive Analysis
- 10.1. Company Market Share Analysis, 2018
- 10.2. Key Industry Developments
- 10.3. Company Profile
- 10.4. GenPact Ltd.
- 10.4.1. Business Overview
- 10.4.2. Segment 1 & Service Offering
- 10.4.3. Overall Revenue
- 10.4.4. Geographic Presence
- 10.4.5. Recent Development
- 10.5. QuintilesIMS
- 10.6. LabCor
- 10.7. PAREXEL
- 10.8. Pharmaceutical Product Development
- 10.9. RESEARCH HOLDINGS INC
- 10.10. PRA Health Sciences
- 10.11. ICON
- 10.12. Charles River
- 10.13. Advent International
- 10.14. WuXi AppTec, Inc.
- 10.15. Pharmaron Beijing Co., Ltd.
- 10.16. Hangzhou Tigermed Consulting Co.,Ltd.
- 10.17. Asymchem Laboratories (Tianjin) Co.,Ltd.
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
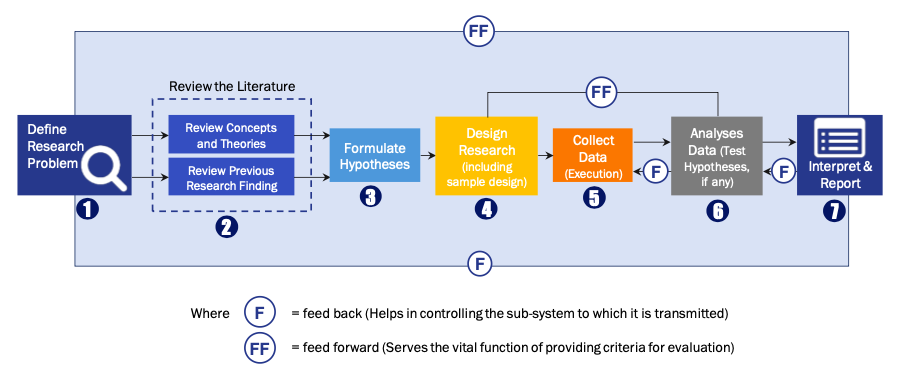
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model