The global non invasive prenatal testing market size was valued at USD 5.1 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 11.23% from 2024 to 2031.
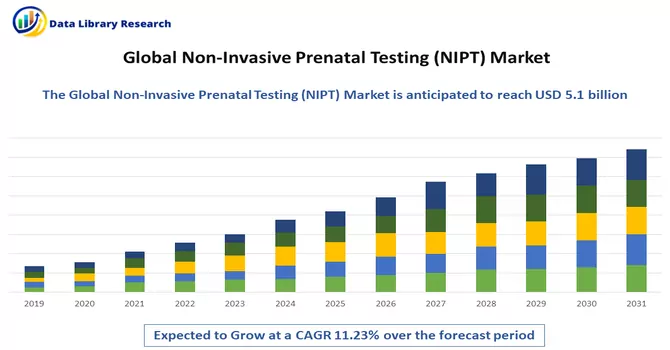
Get Complete Analysis Of The Report - Download Free Sample PDF
The non-invasive prenatal testing (NIPT) market has witnessed significant growth in recent years, driven by advancements in genomic technologies, increasing maternal age, rising awareness about the benefits of early prenatal screening, and growing demand for non-invasive diagnostic techniques. NIPT offers a safer and more accurate alternative to traditional invasive procedures such as amniocentesis and chorionic villus sampling, reducing the risk of miscarriage and other complications associated with these invasive tests.
Additionally, NIPT provides early detection of chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome, enabling healthcare providers to offer timely interventions and support to expectant parents. Moreover, technological innovations, such as the development of cell-free fetal DNA analysis and next-generation sequencing platforms, have further enhanced the accuracy and reliability of NIPT, driving its widespread adoption in prenatal care. As a result, the NIPT market is expected to continue its rapid expansion, fueled by increasing healthcare expenditure, expanding access to prenatal screening services, and growing partnerships between healthcare providers and diagnostic companies. The growth of the non-invasive prenatal testing (NIPT) market is primarily driven by several key factors. Firstly, technological advancements in genomics and molecular biology have significantly improved the accuracy and reliability of NIPT, making it an attractive option for prenatal screening. These advancements have led to the development of highly sensitive and specific assays for detecting fetal chromosomal abnormalities, such as Down syndrome, Edwards syndrome, and Patau syndrome, using cell-free fetal DNA present in maternal blood samples. Secondly, increasing maternal age and the trend towards delayed childbearing have resulted in a higher prevalence of pregnancies at advanced maternal ages, where the risk of chromosomal abnormalities is elevated. As a result, there is a growing demand for non-invasive prenatal testing as an alternative to invasive procedures, such as amniocentesis and chorionic villus sampling, which carry a higher risk of miscarriage and other complications. Additionally, rising awareness among expectant parents and healthcare providers about the benefits of early prenatal screening for detecting fetal abnormalities and enabling timely interventions further drives the adoption of NIPT. Moreover, supportive government initiatives, favorable reimbursement policies, and increasing investments by diagnostic companies in research and development activities contribute to the expansion of the NIPT market. Overall, these factors collectively propel the growth of the non-invasive prenatal testing market worldwide.
Market Segmentation: The non invasive prenatal testing market is Segmented by Test Type (Maternal Plasma DNA Sequencing, Fetal Cells in Maternal Blood Analysis, Biochemical Screening Tests and Other Test Types), Application (Trisomy Detection (e.g., Down syndrome, Edwards syndrome, Patau syndrome), Microdeletion Syndrome Detection and Other Applications), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The report provides revenues and market forecasts in terms of value in USD million for the above segments.
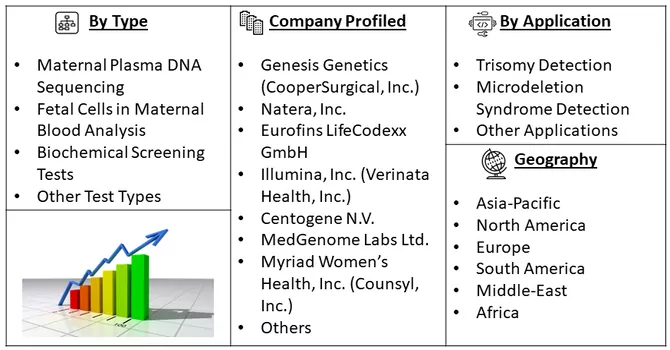
For Detailed Market Segmentation - Download Free Sample PDF
Market trends in the non-invasive prenatal testing (NIPT) sector are characterized by several key developments. Firstly, there is a growing focus on expanding the range of detectable fetal abnormalities beyond common aneuploidies such as Down syndrome, Edwards syndrome, and Patau syndrome. NIPT assays are increasingly being developed to detect microdeletions and microduplications associated with genetic syndromes, as well as single-gene disorders such as cystic fibrosis and sickle cell disease. This trend reflects the increasing demand for comprehensive prenatal screening that provides more detailed information about fetal health. Secondly, there is a shift towards the adoption of cell-free DNA-based testing methods over traditional biochemical markers for prenatal screening. Cell-free DNA analysis offers higher sensitivity and specificity in detecting fetal chromosomal abnormalities, leading to improved accuracy and reduced false-positive rates. Thirdly, there is a growing emphasis on the integration of NIPT into routine prenatal care protocols. Healthcare providers are increasingly recommending NIPT as a first-tier screening option for pregnant women, particularly those at increased risk due to advanced maternal age or other factors. This trend is driven by the growing body of evidence supporting the clinical utility and cost-effectiveness of NIPT compared to traditional screening methods. Lastly, technological advancements continue to drive innovation in the NIPT market, with ongoing research focused on improving test performance, reducing turnaround times, and lowering costs to make NIPT more accessible to a broader population of pregnant women. These trends collectively contribute to the evolution and growth of the non-invasive prenatal testing market.
Market Drivers:
Advancements in Genetic Screening Technology
Continuous advancements in genetic screening technology, particularly in the field of cell-free DNA analysis, have significantly improved the accuracy, sensitivity, and specificity of NIPT assays. Innovations such as next-generation sequencing (NGS) have enabled the detection of a wider range of fetal chromosomal abnormalities and genetic disorders with higher precision. These technological developments have contributed to the growing adoption of NIPT as a preferred method for prenatal screening, driving market growth.
Increasing Maternal Age and Pregnancy Complications
The trend of delayed childbearing, driven by factors such as educational and career pursuits, has led to a rise in maternal age worldwide. Advanced maternal age is associated with an increased risk of chromosomal abnormalities and pregnancy complications, prompting more women to opt for prenatal screening procedures like NIPT. Additionally, the rising prevalence of pregnancy-related conditions such as gestational diabetes and pre-eclampsia has also fueled the demand for early and accurate prenatal diagnosis. As a result, the expanding population of older mothers and high-risk pregnancies acts as a significant driver for the growth of the NIPT market.
Market Restraints:
One of the primary market restraints for the non-invasive prenatal testing (NIPT) market is the high cost associated with these tests. While NIPT offers several advantages over traditional prenatal screening methods, including greater accuracy and reduced risk of miscarriage, the tests tend to be more expensive. This cost factor can limit access to NIPT for certain segments of the population, particularly in regions with limited healthcare resources or inadequate insurance coverage. Additionally, reimbursement policies and coverage limitations by healthcare payers may further restrict the adoption of NIPT among pregnant women. Moreover, despite technological advancements, NIPT may still yield false-positive or false-negative results in some cases, leading to unnecessary anxiety or missed diagnoses. Addressing these cost and reimbursement challenges, along with ongoing efforts to improve the accuracy and reliability of NIPT, will be crucial for overcoming market restraints and enhancing the accessibility of non-invasive prenatal testing.
The COVID-19 pandemic has had a mixed impact on the non-invasive prenatal testing (NIPT) market. On one hand, the pandemic has led to disruptions in healthcare services and resources, causing delays in routine prenatal care and elective procedures, including prenatal testing. This has resulted in a temporary decline in NIPT procedures, as pregnant women may have postponed or skipped these tests due to concerns about visiting healthcare facilities during the pandemic. On the other hand, the pandemic has also highlighted the importance of prenatal testing, especially for high-risk pregnancies, as healthcare providers strive to ensure the well-being of both mother and baby in the face of the virus. Additionally, the shift towards telemedicine and remote healthcare services has facilitated the accessibility of NIPT, allowing pregnant women to consult with healthcare providers and undergo testing from the safety and convenience of their homes. Furthermore, the increased focus on healthcare innovation and research during the pandemic may drive advancements in NIPT technology and adoption in the post-pandemic period. Overall, while the COVID-19 pandemic has posed challenges to the non-invasive prenatal testing market, it has also spurred opportunities for innovation and remote healthcare delivery, potentially shaping the future landscape of prenatal care and testing.
Segmental Analysis:
Maternal Plasma DNA Sequencing Segment is Expected to Witness Significant Growth Over the Forecast Period
Maternal plasma DNA sequencing is a revolutionary technique in non-invasive prenatal testing (NIPT), analyzing cell-free fetal DNA in maternal blood to screen for genetic abnormalities. This method offers high accuracy and lower risk compared to invasive procedures, driving its adoption. The NIPT market is growing due to factors like rising maternal age, increased awareness, and technological advancements, leading to higher demand for early and accurate prenatal screening. As the field evolves, NIPT is expected to become a routine part of prenatal care, offering valuable insights into fetal health.
Hospitals Segment is Expected to Witness Significant Growth Over the Forecast Period
Hospitals play a crucial role in the non-invasive prenatal testing (NIPT) market, serving as key providers of prenatal care and diagnostic services. With the increasing adoption of NIPT as a safer and more accurate alternative to invasive procedures, hospitals are witnessing a growing demand for these tests from expectant parents. Hospitals offering NIPT services benefit from the ability to provide comprehensive prenatal care, including early detection of fetal chromosomal abnormalities such as Down syndrome, Edwards syndrome, and Patau syndrome. This allows healthcare providers to offer timely interventions and support to pregnant women and their families. Furthermore, hospitals often have access to the latest NIPT technologies and expertise, ensuring high-quality testing and interpretation of results. This is critical in providing expectant parents with reliable information about their baby's health, helping them make informed decisions about their pregnancy. As the NIPT market continues to grow, hospitals are expected to play an increasingly important role in expanding access to these tests and improving prenatal care for pregnant women worldwide.
Trisomy Detection (e.g., Down syndrome, Edwards syndrome, Patau syndrome) Segment is Expected to Witness Significant Growth Over the Forecast Period
Trisomy detection, which includes conditions like Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13), is a critical focus area in the non-invasive prenatal testing (NIPT) market. NIPT has emerged as a highly accurate and non-invasive method for screening these chromosomal abnormalities, offering expectant parents a safer alternative to traditional invasive procedures. NIPT for trisomy detection works by analyzing cell-free fetal DNA in the maternal bloodstream, providing high sensitivity and specificity in identifying these conditions. This has led to widespread adoption of NIPT as a routine screening tool for pregnant women, particularly those at increased risk due to factors such as maternal age or abnormal ultrasound findings. The market for NIPT focused on trisomy detection is driven by several factors, including the growing trend of delaying childbirth, which increases the risk of chromosomal abnormalities. Additionally, the high accuracy and early detection capabilities of NIPT have led to its recommendation by healthcare professionals, further boosting its demand. As technology continues to advance, NIPT for trisomy detection is expected to become more accessible and affordable, leading to its integration into routine prenatal care. This will not only improve the detection rates of these chromosomal abnormalities but also provide expectant parents with valuable information to make informed decisions about their pregnancy.
North America Region is Expected to Witness Significant Growth Over the Forecast Period
North America is a key region in the non-invasive prenatal testing (NIPT) market, characterized by a high adoption rate of advanced medical technologies and a strong emphasis on prenatal care. The region has witnessed significant growth in NIPT due to factors such as increasing maternal age, rising awareness about prenatal testing options, and the availability of advanced healthcare infrastructure. In North America, NIPT is widely recognized as a safe and effective method for screening fetal chromosomal abnormalities, including Down syndrome, Edwards syndrome, and Patau syndrome. The region's healthcare systems often recommend NIPT as part of routine prenatal care, leading to high acceptance rates among expectant parents. Furthermore, North America is home to several key players in the NIPT market, including leading manufacturers of NIPT kits and instruments. This has resulted in a competitive market landscape with a wide range of NIPT products and services available to healthcare providers and expectant parents. As the NIPT market in North America continues to evolve, there is a growing focus on expanding the application of NIPT to include additional genetic disorders and microdeletions. This trend is expected to further drive the growth of the NIPT market in the region, making North America a key area of innovation and development in prenatal care.
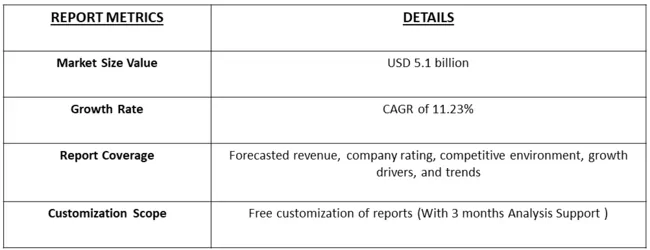
Get Complete Analysis Of The Report - Download Free Sample PDF
The analyzed market exhibits a high degree of fragmentation, primarily attributable to the presence of numerous players operating on both a global and regional scale. The competitive landscape is characterized by a diverse array of companies, each contributing to the overall market dynamics. This fragmentation arises from the existence of specialized solution providers, established industry players, and emerging entrants, all vying for market share. The diversity in market participants is underscored by the adoption of various strategies aimed at expanding the company presence. On a global scale, companies within the studied market are strategically positioning themselves through aggressive expansion initiatives. This often involves entering new geographical regions, targeting untapped markets, and establishing a robust global footprint. The pursuit of global expansion is driven by the recognition of diverse market opportunities and the desire to capitalize on emerging trends and demands across different regions. Simultaneously, at the regional level, companies are tailoring their approaches to align with local market dynamics. Regional players are leveraging their understanding of specific market nuances, regulatory environments, and consumer preferences to gain a competitive edge. This regional focus allows companies to cater to the unique needs of local clientele, fostering stronger market penetration. To navigate the complexities of the fragmented market, companies are implementing a range of strategies. These strategies include investments in research and development to stay at the forefront of technological advancements, mergers and acquisitions to consolidate market share, strategic partnerships for synergies, and innovation to differentiate products and services. The adoption of such multifaceted strategies reflects the competitive nature of the market, with participants continually seeking avenues for growth and sustainability. In essence, the high fragmentation in the studied market not only signifies the diversity of players but also underscores the dynamism and competitiveness that drive ongoing strategic maneuvers. As companies explore various avenues for expansion, the market continues to evolve, presenting both challenges and opportunities for industry stakeholders.
Key Non Invasive Prenatal Testing Companies:
Recent Development:
1) In September 2023, Yourgene Health plc introduced the Yourgene MagBench Automated DNA Extraction Instrument and Kit. This innovative solution, designed for Sage customers in Asia-Pacific and the Middle East, offers a user-friendly, rapid, and cost-effective bench-top robotic workstation for cell-free DNA extraction. Specifically tailored for Yourgene’s Sage 32 NIPT Workflow, this comprehensive solution empowers clinical laboratories to provide precise and competitive non-invasive prenatal testing (NIPT) services, ensuring efficiency from sample to report.
2) In August 2022, Natera, Inc. announced at the Canaccord Genuity 42nd Annual Growth Conference in Boston that it had proactively initiated the FDA pre-submission process for its Panorama non-invasive prenatal test (NIPT) through the Q-Sub process. The filing, submitted in June 2022, focuses on fetal chromosomal aneuploidies and targets 22q11.2 deletion syndrome. This proactive step demonstrates Natera's commitment to navigating regulatory pathways and seeking approval for its advanced Panorama NIPT, highlighting its dedication to enhancing prenatal testing options.
Q1. What was the Non-Invasive Prenatal Testing (NIPT) Market size in 2023?
Non-Invasive Prenatal Testing (NIPT) Market the global non invasive prenatal testing market size was valued at USD 5.1 billion in 2023.
Q2. At what CAGR is the Non-Invasive Prenatal Testing (NIPT) market projected to grow within the forecast period?
Non-Invasive Prenatal Testing (NIPT) Market is expected to grow at a compound annual growth rate (CAGR) of 11.23% over the forecast period.
Q3. What are the factors driving the Non-Invasive Prenatal Testing (NIPT) Market?
Key factors that are driving the growth include the Advancements in Genetic Screening Technology and Increasing Maternal Age and Pregnancy Complications.
Q4. Who are the key players in Non-Invasive Prenatal Testing (NIPT) Market?
Some key players operating in the market include
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS

Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model
