Increasing the number of medicine and drug in healthcare industry that demand for innovative drugs thus needful to large pharmaceutical companies try to remain competitive by conducting faster drug development with corresponding cost containment. Additionally, after the regulatory approval of the drug, these companies are left with relatively less time to deliver the drug within the market in substantial quantity. These factors are leading to greater outsourcing of producing facilities to pharmaceutical contract manufacturers.
Moreover, the influx of small and virtual start-ups with negligible manufacturing capacity is augmenting the worldwide pharmaceutical contract manufacturing market. The rising number of U.S. FDA-approved manufacturing facilities in developing countries is additionally working in favour of the expansion of the market.
The capacity utilization issues and lack of producing standardization are affecting the profitability of contract manufacturing organizations. This, in turn, is hampering the general revenue generation of the market.
Covid-19 Impact on Pharmaceutical Contract Development and Manufacturing Market:
In addition, the current Global Market study offers a detailed analysis of the current COVID-19 pandemic impact on the market growth and its influence on the future growth of the Global Market. The recently published report demonstrates the elevation within the demand for the healthcare sector. The healthcare manufacturers have experienced future also as short term effect which incorporates supply shortages, panic buying, and stocking, regulation changes as short-term whereas approval delays and possible trend variations in consumption might be perceived as long-term impacts of COVID-19 on the health and pharmaceutical market.
The increasing need for a cure has pushed vaccine research and makers to the limit. In addition to the present, panic conditions have already spurred the demand for several healthcare products and services which are discussed intimately during this report. Moreover, the impact of this pandemic on overall market revenue for the base year 2020 and its projection up to 2027 is provided in detail in this report.
Pharmaceutical Contract Development and Manufacturing Market Segment Overview
By Services segment, Research holds the major market share in estimated time period. Also, Globalization of clinical trials, increasing government funding for healthcare and a rise within the number of clinical sites are expected to drive the demand research segment.
By End-users, the generic pharma accounted for the most important share of this market in 2020. This share is attributed to the rising R&D efforts by these companies.
Market Analysis, Insights and Forecast – By Services
- Manufacturing
- API/Bulk Drugs
- Advanced Drug Delivery Formulations Packaging
- Packaging
- Finished Dose Formulations
- Solid Formulations
- Liquid Formulations
- Semi-solid Formulations
- Research
- Oncology
- Vaccines
- Inflammation & Immunology
- Cardiology
- Neuroscience
- Others
· Big Pharma
· Small Pharma
· Generic Pharma
· Contract Research Organisations (CROs)
· Other
Pharmaceutical Contract Development and Manufacturing Market Regional Overview
Region-wise, in terms of regions, North America as well as Europe holds the market share within the global pharmaceutical contract manufacturing market. The expansion of those regions is supplemented by the growing geriatric population and high uptake of biologics. The increasing research activities by biotechnology companies and therefore the rising demand for generics also are contributing to the expansion of the regions. The U.S. is going to be a serious revenue contributor within the North America market. The Asia Pacific region is estimated to emerge as a lucrative market during the forecast period due to the improving healthcare infrastructure and increasing favourable government initiatives.
Pharmaceutical Contract Development and Manufacturing Market, By Geography
· North America (US & Canada)
· Europe (UK, Germany, France, Italy, Spain, & Rest of Europe)
· Asia-Pacific (Japan, China, India, Australia, & South Korea, & Rest of Asia-Pacific)
· LAMEA (Brazil, Saudi Arabia, UAE & Rest of LAMEA)
Pharmaceutical Contract Development and Manufacturing Market Competitor overview
Some key developments and strategies adopted by manufacturers in the Pharmaceutical Contract Development and Manufacturing are highlighted below.
· In January 2019, Lambda, headquartered in India, acquired the US-based Novum Pharmaceutical Research Services.
Pharmaceutical Contract Development and Manufacturing Market, Key Players
· Thermo Fisher
· Catalent, Inc.
· Lonza Group Ltd.
· Recipharm AB
· AbbVie Inc.
· Aenova Group
· Almac Group
· Siegfried Holding AG
· Ingelheim International
1. Introduction
- 1.1. Research Scope
- 1.2. Market Segmentation
- 1.3. Research Methodology
- 1.4. Definitions and Assumptions
2. Executive Summary
3. Market Dynamics
- 3.1. Market Drivers
- 3.2. Market Restraints
- 3.3. Market Opportunities
4. Key Insights
- 4.1. Key Emerging Trends – For Major Countries
- 4.2. Latest Technological Advancement
- 4.3. Regulatory Landscape
- 4.4. Industry SWOT Analysis
- 4.5. Porters Five Forces Analysis
5. Global Pharmaceutical Contract Development and Manufacturing Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 5.1. Key Findings / Summary
- 5.2. Market Analysis, Insights and Forecast – By Product And Services
- 5.2.1. Manufacturing
- 5.2.1.1. API/Bulk Drugs
- 5.2.1.2. Advanced Drug Delivery Formulations Packaging
- 5.2.1.3. Packaging
- 5.2.1.4. Finished Dose Formulations
- 5.2.1.5. Solid Formulations
- 5.2.1.6. Liquid Formulations
- 5.2.1.7. Semi-solid Formulations
- 5.2.2. Research
- 5.2.2.1. Oncology
- 5.2.2.2. Vaccines
- 5.2.2.3. Inflammation & Immunology
- 5.2.2.4. Cardiology
- 5.2.2.5. Neuroscience
- 5.2.2.6. Others
- 5.2.1. Manufacturing
- 5.3.1. Big Pharma
- 5.3.2. Small Pharma
- 5.3.3. Generic Pharma
- 5.3.4. Contract Research Organisations (CROs)
- 5.3.5. Other
- 5.4.1. North America
- 5.4.2. Europe
- 5.4.3. Asia Pacific
- 5.4.4. Latin America, Middle East, and Africa
6. North America Pharmaceutical Contract Development and Manufacturing Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 6.1. Key Findings / Summary
- 6.2. Market Analysis, Insights and Forecast – By Product And Services
- 6.2.1. Manufacturing
- 6.2.1.1. API/Bulk Drugs
- 6.2.1.2. Advanced Drug Delivery Formulations Packaging
- 6.2.1.3. Packaging
- 6.2.1.4. Finished Dose Formulations
- 6.2.1.5. Solid Formulations
- 6.2.1.6. Liquid Formulations
- 6.2.1.7. Semi-solid Formulations
- 6.2.2. Research
- 6.2.2.1. Oncology
- 6.2.2.2. Vaccines
- 6.2.2.3. Inflammation & Immunology
- 6.2.2.4. Cardiology
- 6.2.2.5. Neuroscience
- 6.2.2.6. Others
- 6.2.1. Manufacturing
- 6.3.1. Big Pharma
- 6.3.2. Small Pharma
- 6.3.3. Generic Pharma
- 6.3.4. Contract Research Organisations (CROs)
- 6.3.5. Other
- 6.4.1. U.S.
- 6.4.2. Canada
7. Europe Pharmaceutical Contract Development and Manufacturing Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 7.1. Key Findings / Summary
- 7.2. Market Analysis, Insights and Forecast – By Product And Services
- 7.2.1. Manufacturing
- 7.2.1.1. API/Bulk Drugs
- 7.2.1.2. Advanced Drug Delivery Formulations Packaging
- 7.2.1.3. Packaging
- 7.2.1.4. Finished Dose Formulations
- 7.2.1.5. Solid Formulations
- 7.2.1.6. Liquid Formulations
- 7.2.1.7. Semi-solid Formulations
- 7.2.2. Research
- 7.2.2.1. Oncology
- 7.2.2.2. Vaccines
- 7.2.2.3. Inflammation & Immunology
- 7.2.2.4. Cardiology
- 7.2.2.5. Neuroscience
- 7.2.2.6. Others
- 7.2.1. Manufacturing
- 7.3.1. Big Pharma
- 7.3.2. Small Pharma
- 7.3.3. Generic Pharma
- 7.3.4. Contract Research Organisations (CROs)
- 7.3.5. Other
- 7.4.1. UK
- 7.4.2. Germany
- 7.4.3. France
- 7.4.4. Italy
- 7.4.5. Spain
- 7.4.6. Russia
- 7.4.7. Rest of Europe
8. Asia Pacific Pharmaceutical Contract Development and Manufacturing Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 8.1. Key Findings / Summary
- 8.2. Market Analysis, Insights and Forecast – By Product And Services
- 8.2.1. Manufacturing
- 8.2.1.1. API/Bulk Drugs
- 8.2.1.2. Advanced Drug Delivery Formulations Packaging
- 8.2.1.3. Packaging
- 8.2.1.4. Finished Dose Formulations
- 8.2.1.5. Solid Formulations
- 8.2.1.6. Liquid Formulations
- 8.2.1.7. Semi-solid Formulations
- 8.2.2. Research
- 8.2.2.1. Oncology
- 8.2.2.2. Vaccines
- 8.2.2.3. Inflammation & Immunology
- 8.2.2.4. Cardiology
- 8.2.2.5. Neuroscience
- 8.2.2.6. Others
- 8.2.1. Manufacturing
- 8.3.1. Big Pharma
- 8.3.2. Small Pharma
- 8.3.3. Generic Pharma
- 8.3.4. Contract Research Organisations (CROs)
- 8.3.5. Other
- 8.4.1. China
- 8.4.2. India
- 8.4.3. Japan
- 8.4.4. Australia
- 8.4.5. South East Asia
- 8.4.6. Rest of Asia Pacific
9. Latin America, Middle East, and Africa Pharmaceutical Contract Development and Manufacturing Market Analysis (USD Billion), Insights and Forecast, 2020-2027
- 9.1. Key Findings / Summary
- 9.2. Market Analysis, Insights and Forecast – By Product And Services
- 9.2.1. Manufacturing
- 9.2.1.1. API/Bulk Drugs
- 9.2.1.2. Advanced Drug Delivery Formulations Packaging
- 9.2.1.3. Packaging
- 9.2.1.4. Finished Dose Formulations
- 9.2.1.5. Solid Formulations
- 9.2.1.6. Liquid Formulations
- 9.2.1.7. Semi-solid Formulations
- 9.2.2. Research
- 9.2.2.1. Oncology
- 9.2.2.2. Vaccines
- 9.2.2.3. Inflammation & Immunology
- 9.2.2.4. Cardiology
- 9.2.2.5. Neuroscience
- 9.2.2.6. Others
- 9.2.1. Manufacturing
- 9.3.1. Big Pharma
- 9.3.2. Small Pharma
- 9.3.3. Generic Pharma
- 9.3.4. Contract Research Organisations (CROs)
- 9.3.5. Other
- 9.4.1. Brazil
- 9.4.2. Saudi Arabia
- 9.4.3. UAE
- 9.4.4. Rest of LAMEA
10. Competitive Analysis
- 10.1. Company Market Share Analysis, 2018
- 10.2. Key Industry Developments
- 10.3. Company Profile
- 10.4. Thermo Fisher
- 10.4.1. Business Overview
- 10.4.2. Segment 1 & Service Offering
- 10.4.3. Overall Revenue
- 10.4.4. Geographic Presence
- 10.4.5. Recent Development
- 10.5. Catalent, Inc.
- 10.6. Lonza Group Ltd.
- 10.7. Recipharm AB
- 10.8. AbbVie Inc.
- 10.9. Aenova Group
- 10.10. Almac Group
- 10.11. Siegfried Holding AG
- 10.12. Ingelheim International
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
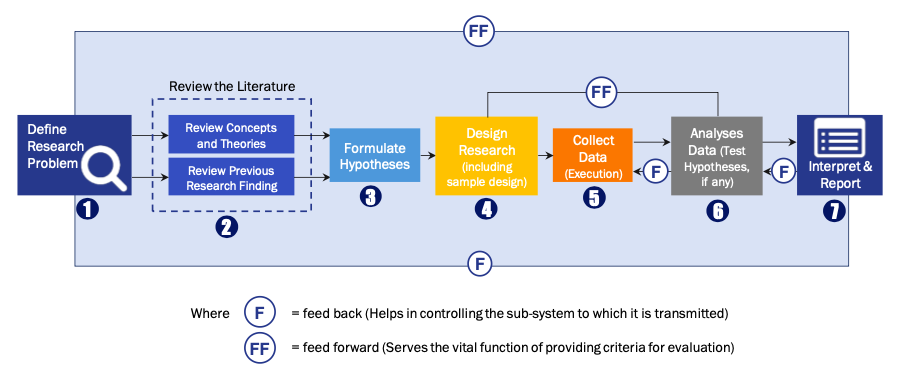
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model