The RNAi therapeutics and technology market is estimated to grow from USD 2.98 billion in 2025 to USD 8.18 billion by 2032, growing at a CAGR of 15.21% over the forecast period.
Get Complete Analysis Of The Report - Download Free Sample PDF
The RNAi Therapeutics Market refers to the global industry focused on developing and commercializing therapies that use RNA interference (RNAi)—a natural cellular process that silences specific genes—to treat various diseases. These therapies work by targeting and “switching off” faulty or harmful genes responsible for conditions like cancers, genetic disorders, viral infections, and liver diseases. The market includes small interfering RNAs (siRNAs), microRNAs (miRNAs), and related delivery systems. Growing interest in precision medicine, rising investments in biotechnology, and recent regulatory approvals are driving market growth. As RNAi therapeutics offer a novel, highly targeted way to treat previously hard-to-manage diseases, the market is expanding rapidly, attracting pharmaceutical companies, research institutions, and investors worldwide.
The RNAi Therapeutics Market is experiencing strong growth driven by advances in gene-silencing technologies, increasing investments in biotech R&D, and the successful approval of RNAi-based drugs like Onpattro and Givlaari. A major trend is the development of targeted delivery systems, such as lipid nanoparticles and conjugates, which improve the precision and safety of RNAi therapies. There is also growing interest in rare and genetic diseases, where RNAi offers highly specific treatment options with fewer side effects. Collaborations between biotech startups and large pharmaceutical companies are accelerating clinical pipelines. Additionally, the expansion of RNAi applications beyond liver diseases into areas like oncology, neurology, and infectious diseases signals a broader therapeutic potential, further fueling market momentum.
Segmentation: The RNAi Technology Market Report is Segmented by Molecule Type (siRNA, Mirna, and Others), Route of Administration (Intravenous, Intradermal, Pulmonary Delivery, Intrathecal, and Others), Indication (Acute Hepatic Porphyria (AHP), Hereditary Transthyretin-Mediated Amyloidosis (hATTR), Hypercholesterolemia, and Others), Delivery Technology (Lipid Nanoparticles Including Ionizable LNPs and Liposomes, and Others), End User (Pharmaceutical & Biotechnology Companies, Cdmos, and Others), and Geography (North America, and More). The Market Forecasts are Provided in Terms of Value (USD).
For Detailed Market Segmentation - Get a Free Sample PDF
Market Drivers:
The successful clinical validation and subsequent regulatory approval of small interfering RNA (siRNA) therapies—such as those for rare genetic and cardiometabolic disorders—is the primary catalyst fueling market growth. These approvals have transformed RNAi from a promising research tool into a commercially viable therapeutic modality. For instance, in July 2025, Avidity Biosciences, Inc. announced the completion of enrollment in its global Phase 3 HARBOR™ clinical trial evaluating delpacibart etedesiran (del-desiran) for patients with myotonic dystrophy type 1 (DM1). This trial is the first global Phase 3 study in DM1, with topline results expected in the second quarter of 2026. The progress of this large-scale Phase 3 trial highlights growing regulatory confidence in siRNA-based drugs, reflecting successful clinical outcomes and advancing RNAi therapies toward broader approval and adoption.
Clinical efficacy in reducing target protein production, coupled with durable effects, has significantly boosted investor and pharmaceutical confidence. This success attracts substantial R&D funding, fosters strategic alliances between biotech innovators and major pharmaceutical companies, and rapidly accelerates pipeline development. The positive regulatory precedent established by the first-to-market products streamlines the path for new candidates, rapidly expanding the therapeutic scope beyond rare diseases into high-prevalence indications.
The historical hurdle of safe and effective in vivo delivery is a key market driver. Advances in delivery technology, particularly the development of N-acetylgalactosamine (GalNAc) conjugates and improved Lipid Nanoparticles (LNPs), have revolutionized RNAi therapeutics. GalNAc conjugates enable precise targeting of the liver, leading to highly effective and convenient subcutaneous dosing for hepatic diseases. LNPs offer a robust platform for systemic delivery to other tissues. These sophisticated systems significantly enhance the stability of the RNA molecule, improve cellular uptake, and crucially, minimize off-target effects and immune responses, thereby improving the drug's therapeutic index and patient compliance.
Furthermore, the recent mergers, and partnerships between major players ate further fueling the growth of above market. For instance, in November 2024, Sarepta Therapeutics, Inc. entered into an exclusive global licensing and collaboration agreement with Arrowhead Pharmaceuticals, Inc., securing worldwide rights to multiple clinical, preclinical, and discovery-stage RNAi programs targeting rare genetic diseases affecting the muscles, central nervous system (CNS), and lungs. This partnership significantly strengthened Sarepta’s early and mid-stage pipeline, expanding its focus beyond Duchenne muscular dystrophy and limb-girdle muscular dystrophies into adjacent therapeutic areas. As part of the agreement, Sarepta CEO Doug Ingram was appointed to Arrowhead’s Board of Directors, reinforcing strategic alignment and collaboration between the two companies.
Market Restraints:
The key restraints in the RNAi therapeutics market is the challenge of efficient and targeted delivery of RNA molecules to specific tissues and cells. Despite advances like lipid nanoparticles (LNPs), delivery outside the liver remains difficult, limiting the range of treatable conditions. Additionally, there is concern over off-target effects, where RNAi molecules may unintentionally silence non-target genes, potentially leading to adverse reactions or reduced treatment effectiveness. Stability of RNA in the bloodstream and immune system activation are also ongoing hurdles. These scientific and technical barriers can delay drug development, increase costs, and hinder regulatory approvals. Overcoming these limitations is critical for expanding RNAi's clinical applications and ensuring broader market adoption across diverse therapeutic areas.
The RNAi market is poised to have a transformative socio-economic impact by offering highly precise treatments for formerly "undruggable" diseases, especially rare genetic and chronic conditions. By targeting the root cause (disease-causing mRNA), these therapies can shift care from palliative management to disease modification, significantly improving patient quality of life and longevity. However, the high upfront cost of these innovative treatments creates a complex payer challenge, potentially widening the healthcare equity gap. On the economic side, the sector fosters high-skill job creation in specialized biotech manufacturing and R&D, while successful pipeline development promises substantial long-term savings by reducing the massive lifetime costs associated with chronic disease management.
Segmental Analysis:
The small interfering RNA (siRNA) segment is projected to experience significant growth due to its proven ability to selectively silence disease-causing genes. siRNA-based drugs like Onpattro and Givlaari have already demonstrated strong clinical success, validating the technology. With expanding research and regulatory support, more siRNA therapies are entering clinical trials, targeting a wide range of diseases from rare genetic disorders to cancers and viral infections. Advancements in delivery technologies, especially lipid nanoparticles, are enhancing siRNA stability and effectiveness in the body. As biotech companies and pharmaceutical firms invest heavily in siRNA pipelines, this segment is expected to become a dominant force in the RNAi therapeutics market over the coming years, offering highly targeted and innovative treatment options.
The hATTR segment is expected to see strong growth within the RNAi therapeutics market, primarily due to the success of pioneering drugs like Onpattro, the first FDA-approved RNAi therapy for this condition. hATTR is a rare, life-threatening disease caused by mutations in the transthyretin (TTR) gene, leading to protein buildup in nerves and organs. RNAi treatments offer a highly targeted solution by silencing the faulty gene, significantly improving patient outcomes. Increased awareness, better diagnosis, and rising demand for effective treatments for rare diseases are fueling investment and innovation in this segment. As more clinical data supports RNAi efficacy in hATTR, additional approvals and expanded indications are expected, further driving market growth and patient adoption worldwide.
Lipid nanoparticles (LNPs), especially ionizable LNPs, are playing a critical role in the success of RNAi therapeutics, and this segment is expected to grow rapidly. These advanced delivery systems protect RNA molecules from degradation, ensure targeted delivery to specific cells (especially in the liver), and minimize side effects. The success of LNPs in mRNA vaccines during the COVID-19 pandemic has boosted confidence and interest in their use for RNAi as well. Ionizable LNPs, in particular, offer better safety profiles and efficiency, making them the preferred method for systemic RNA delivery. As new RNAi therapies move through clinical development, the demand for effective delivery technologies like ionizable LNPs will continue to surge, driving innovation and adoption in this segment.
Pharmaceutical and biotechnology companies are expected to dominate the RNAi therapeutics market as they lead in research, development, and commercialization of innovative gene-silencing therapies. These companies are heavily investing in RNAi platforms, expanding clinical pipelines, and forming strategic collaborations to accelerate drug development. The growing demand for personalized medicine and targeted therapies has pushed pharma and biotech firms to focus on RNAi due to its high specificity and reduced risk of off-target effects. Successful case studies, such as Alnylam Pharmaceuticals and its approved drugs, have further validated RNAi’s potential, encouraging more companies to enter the space. With increasing funding, R&D activity, and regulatory support, this segment is well-positioned for significant growth throughout the forecast period.
North America, particularly the United States, is expected to witness substantial growth in the RNAi therapeutics market due to its strong biotech ecosystem, advanced healthcare infrastructure, and robust funding for life sciences innovation. The region is home to leading RNAi companies, including pioneers like Alnylam Pharmaceuticals and Arrowhead Pharmaceuticals, which are driving major developments and clinical trials.
Favorable regulatory pathways from the FDA, growing awareness of genetic disorders, and a well-established reimbursement framework are contributing to increased adoption of RNAi-based therapies. For instance, in September 2025, Arrowhead Pharmaceuticals, Inc. filed for regulatory clearance to start a Phase 1/2a clinical trial of ARO-MAPT, its investigational RNA interference (RNAi) therapy targeting tauopathies, including Alzheimer’s disease. Alzheimer’s, the leading cause of dementia, affects an estimated 32 million people globally and is characterized by progressive cognitive and functional decline. The disease is part of a group of neurodegenerative disorders known as tauopathies, which involve abnormal accumulation of tau protein tangles in neurons. This trial marks an important step in evaluating ARO-MAPT’s potential to treat these devastating conditions through RNAi-based gene silencing. Additionally, significant government and private investments in genetic research and precision medicine further support the market's expansion. As new drugs receive approval and awareness grows, North America is likely to remain at the forefront of RNAi innovation and adoption.
To Learn More About This Report - Request a Free Sample Copy
The competitive landscape is dominated by a few established players, most notably Alnylam Pharmaceuticals, which holds a significant first-mover advantage and a portfolio of approved products. Competition centers fiercely on proprietary delivery technologies (like GalNAc conjugates and advanced LNPs) and intellectual property rights, leading to numerous cross-industry collaborations and licensing agreements between specialized biotechs and large pharmaceutical companies (e.g., Roche, Novo Nordisk). Emerging players focus on expanding RNAi application into new therapeutic areas, such as oncology and neurology, intensifying the race to secure novel targets and delivery platforms that can safely address non-hepatic tissues. The market is moderately concentrated but highly dynamic, driven by continuous innovation.
The major players for this market are:
Recent Developments:
Q1. What are the main growth driving factors for this market?
The market is primarily driven by the proven clinical validation of first-to-market small interfering RNA (siRNA) drugs, which has significantly increased investor and pharmaceutical company confidence. Key technological advancements, particularly in delivery systems like GalNAc conjugates and lipid nanoparticles (LNPs), are crucial as they enhance drug safety, durability, and patient convenience. Additionally, the rising prevalence of chronic and genetic disorders creates a growing demand for these highly specific, targeted therapeutic approaches. Strategic partnerships between biotech innovators and global pharma leaders further accelerate research, commercialization, and the expansion of the clinical pipeline into major disease areas like cardiovascular disease and oncology.
Q2. What are the main restraining factors for this market?
Major restraining factors include persistent challenges in safe and effective drug delivery, despite recent advances. Specifically, concerns remain regarding potential off-target effects (silencing unintended genes) and the risk of triggering an innate immune response, which necessitates rigorous regulatory scrutiny and prolongs clinical timelines. Furthermore, the high capital investment required for specialized oligonucleotide manufacturing and the complex, competitive patent landscape surrounding proprietary delivery chemistries (like ionizable lipids) create significant financial and legal hurdles for market entry and sustained growth.
Q3. Who are the top major players for this market?
The RNAi therapeutics market is led by companies that have successfully commercialized approved siRNA therapies and possess strong proprietary delivery platforms. Top major players include Alnylam Pharmaceuticals, a pioneer with multiple approved RNAi drugs (e.g., Onpattro, Givlaari), and Arrowhead Pharmaceuticals, known for its TRiM™ platform. Global pharmaceutical giants like Novartis and Novo Nordisk are also major players, having strategically acquired or partnered to incorporate RNAi technology into their portfolios, solidifying the market through significant investments and commercialization efforts.
Q4. Which country is the largest player?
The United States is the largest individual country player and the primary driver of the North American market, which collectively holds the largest global revenue share (around 43%). This dominance is attributed to the presence of a mature and robust biotechnology ecosystem, a vast pool of venture capital and research funding, and a highly supportive regulatory environment (e.g., the FDA) that has accelerated the development and early approval of pioneering RNAi therapeutics, establishing global commercial and research benchmarks.
Q5. Which segment is expected to witness high growth?
By molecule type, the small interfering RNA (siRNA) segment is expected to witness high growth and currently dominates the market due to its established clinical success and compatibility with advanced delivery systems like GalNAc conjugates and LNPs. By application, the segment targeting Oncology is anticipated to expand rapidly, leveraging RNAi's ability to silence historically "undruggable" disease-driving genes. Regionally, the Asia-Pacific market is projected to grow the fastest, fueled by rising healthcare investment, expanding biotechnology infrastructure, and supportive government policies.
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
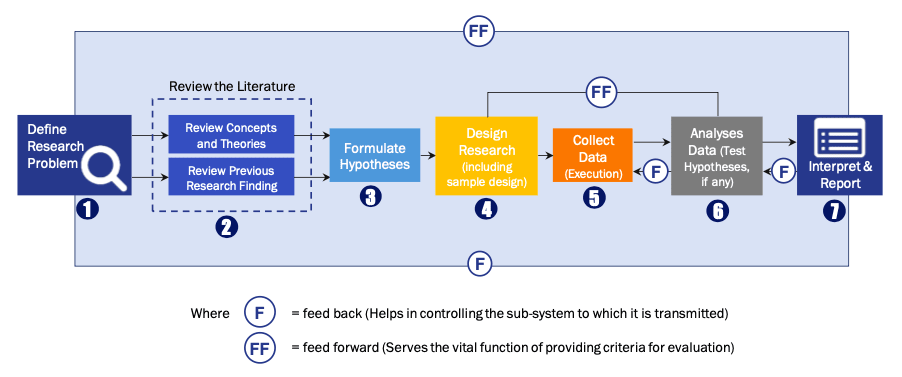
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model