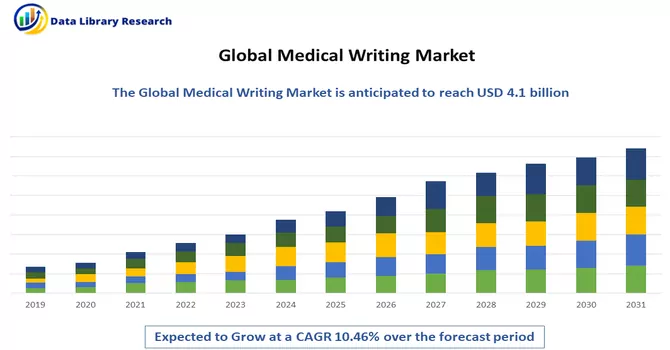
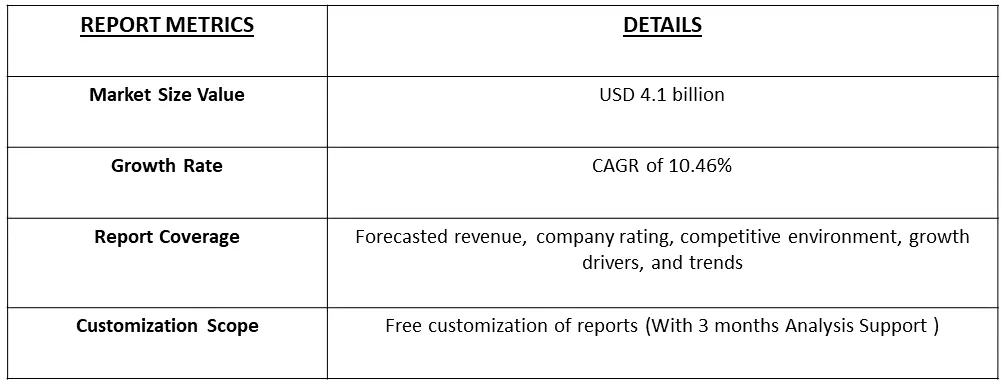
The global medical writing market size was valued at USD 4.1 billion in 2023 and is expected to expand at a CAGR of 10.46% from 2023 to 2031.
Get Complete Analysis Of The Report - Download Free Sample PDF
Medical writing involves the creation of various types of documents within the healthcare and life sciences domains. These documents are critical for communicating scientific and medical information to diverse audiences, including healthcare professionals, regulatory agencies, patients, and the general public. Medical writers play a crucial role in translating complex scientific data and research findings into clear, accurate, and comprehensible documents. The types of documents created by medical writers include clinical trial protocols, regulatory submission documents, scientific manuscripts, patient education materials, product labels, and other materials that contribute to the dissemination of medical knowledge and information. Medical writing requires a thorough understanding of medical and scientific concepts, as well as proficiency in writing and communication skills.
The medical writing market is experiencing robust growth driven by several factors. As clinical research activities in the pharmaceutical and biotechnology sectors become more complex, the demand for comprehensive documentation, including clinical trial protocols and regulatory submissions, is on the rise. Stringent regulatory requirements in healthcare and pharmaceuticals contribute to the need for precise and compliant documentation. With an increasing number of drug approvals and regulatory submissions, skilled medical writers play a pivotal role in creating well-structured submission dossiers. Globalization of clinical trials and collaboration among international teams amplify the demand for medical writers who can meet global standards. The emphasis on evidence-based medicine and accurate documentation fuels the need for medical writers to create scientific manuscripts and literature reviews. Outsourcing medical writing services to specialized firms is a growing trend. Finally, the continuous advancements in therapeutics and healthcare interventions necessitate up-to-date documentation, making medical writers crucial contributors to scientific literature and educational materials.
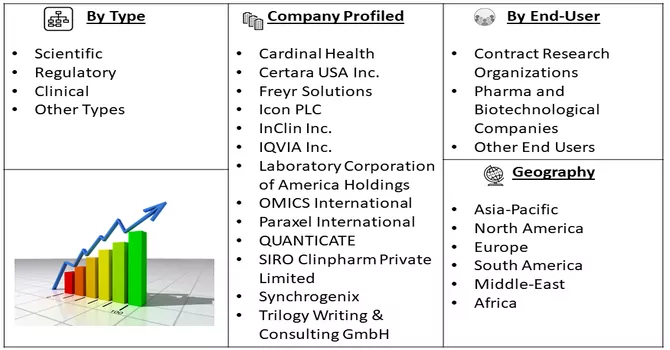
Market Segmentation: The Global Medical Writing Market is Segmented by Type (Scientific, Regulatory, Clinical, and Other Types), End User (Contract Research Organizations, Pharma and Biotechnological Companies, and Other End Users), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The market size and forecasts are provided in terms of value (in USD million) for the above-mentioned segments.
For Detailed Market Segmentation - Download Free Sample PDF
The medical writing market is witnessing several notable trends that shape its dynamics. One key trend is the increasing adoption of artificial intelligence (AI) and automation in medical writing processes. AI tools are being utilized to enhance the efficiency of literature reviews, data analysis, and report generation, contributing to faster and more accurate medical writing. Another trend involves the growing emphasis on real-world evidence (RWE) generation, driven by the need for comprehensive data to support healthcare decisions. As a result, medical writers are increasingly involved in creating RWE documents and publications. The rise of remote and virtual clinical trials has also impacted medical writing practices, with a shift toward digital platforms for documentation and collaboration. Furthermore, there is a notable trend towards transparency and open access in medical research, influencing the way medical writers approach publication practices. The incorporation of multimedia elements, such as infographics and videos, into medical writing is gaining traction for more effective communication. Overall, these trends reflect the evolving landscape of medical writing, driven by technological advancements and changing preferences in the healthcare and research sectors.
Market Drivers:
Growing R&D expenditure and Development of New Therapeutics
The medical writing market is witnessing robust growth driven by the increasing emphasis on research and development (R&D) activities and the continual development of new therapeutics. As the pharmaceutical and life sciences industries expand their R&D initiatives to discover innovative treatments and drugs, there is a growing demand for skilled medical writers who can effectively communicate complex scientific information. The escalating investment in R&D endeavours by pharmaceutical companies, biotech firms, and academic institutions contributes significantly to the expanding landscape of medical writing services. Additionally, the continuous development of novel therapeutics, including biologics, gene therapies, and precision medicine, necessitates clear and accurate documentation, further propelling the demand for proficient medical writers. This trend is expected to persist as the healthcare sector continues to advance, emphasizing the pivotal role of medical writing in translating scientific discoveries into accessible and comprehensible content.
Increasing Contract Research Organizations and Outsourcing Services
The medical writing landscape is experiencing notable growth due to the increasing prominence of Contract Research Organizations (CROs) and the growing trend of outsourcing services in the healthcare and pharmaceutical sectors. Contract Research Organizations play a pivotal role in conducting clinical trials and research studies on behalf of pharmaceutical companies and other stakeholders. As these organizations handle diverse aspects of drug development, including regulatory submissions and documentation, there is a heightened demand for specialized medical writing services. Outsourcing medical writing tasks to CROs enables pharmaceutical companies to streamline their operations, reduce costs, and access the expertise of skilled medical writers. This trend is particularly significant as the pharmaceutical industry continues to expand its global footprint, relying on external partners for various aspects of drug development. The surge in outsourcing services and collaborations with CROs underscores the essential role of medical writing in efficiently communicating scientific findings and regulatory information across the pharmaceutical and healthcare ecosystem.
Market Restraints:
Lack of Skilled Professionals for Medical Writing
The medical writing sector faces a challenge related to the lack of skilled professionals. As the demand for medical writing services continues to grow, there is a noticeable shortage of qualified individuals with expertise in translating complex scientific data into clear and concise documents. Medical writing requires a unique skill set that combines a thorough understanding of scientific concepts with effective communication abilities. The intricacies of regulatory documentation, clinical trial reports, and other medical writing tasks necessitate professionals with a keen eye for detail, scientific acumen, and proficiency in relevant guidelines. The scarcity of skilled medical writers can potentially lead to delays in document preparation, hinder effective communication within the healthcare and pharmaceutical industries, and impact the overall quality of medical publications. Addressing this shortage through targeted training and education initiatives is crucial for sustaining the growth and integrity of the medical writing market.
COVID-19 Impact on Medical Writing Market:
The medical writing sector underwent substantial changes during the pandemic, responding to an increased demand for efficient information dissemination. Initially, projections suggested a decline in submissions to medical journals at the pandemic's onset. However, by the end of 2020, several high-impact journals experienced noteworthy surges in submissions. A study titled "Pandemic Publishing Medical journals strongly speed up their publication process for COVID-19," published in August 2020, highlighted that the scholarly publishing industry remarkably expedited the release of information related to coronaviruses. This acceleration was particularly observed in articles pertaining to coronaviruses. Hence, the COVID-19 pandemic is poised to exert a substantial influence on the medical writing market, driven by these evolving dynamics.
Segmental Analysis:
Regulatory Medical Writing Segment is Expected to Witness Significant Growth Over the Forecast Period
Regulatory medical writing is a specialized discipline within the broader field of medical writing that focuses on producing documents for regulatory authorities, such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These documents play a crucial role in the drug development and approval process, providing comprehensive and accurate information about the safety, efficacy, and quality of pharmaceutical products. Regulatory medical writers collaborate closely with subject matter experts, clinical teams, and regulatory affairs professionals to prepare documents like clinical study reports, investigator brochures, and regulatory submissions. The precision and clarity of these documents are paramount, as they influence regulatory decisions and contribute to the overall success of a drug development program. Adherence to regulatory guidelines and standards is a key aspect of regulatory medical writing to ensure that submissions meet the stringent requirements of health authorities.
In March 2023, PROMETRIKA, LLC expanded its strategic regulatory consulting services, led by Aileen Ryan, MS, Senior Regulatory Advisor. Aileen Ryan, who has been providing part-time regulatory consulting services to PROMETRIKA’s client companies for the past 2 years, has now joined the company on a full-time basis. With her leadership, PROMETRIKA aims to provide comprehensive and expert regulatory writing services, catering to diverse needs such as submissions in the US, Canada, EU, and Japan. This move signifies a commitment to offering high-quality regulatory consulting services, ensuring clients receive strategic and effective support in navigating the complexities of regulatory processes. Thus, such developments are expected to fuel the growth of the studied segment over the forecast period.
Contract Research Organizations Segment is Expected to Witness Significant Growth Over the Forecast Period
Contract Research Organizations (CROs) play a crucial role in the field of medical writing, contributing to the efficiency and success of clinical research and regulatory processes. CROs specializing in medical writing offer a range of services to pharmaceutical, biotechnology, and medical device companies. These services include the preparation of various regulatory documents, clinical study reports, protocols, investigator brochures, and other essential documents required for regulatory submissions. By engaging CROs for medical writing, companies can benefit from the expertise and experience of skilled medical writers, ensuring the production of high-quality, compliant, and scientifically rigorous documents. This collaborative approach allows organizations to streamline their clinical development processes, adhere to regulatory standards, and focus on core research activities while leveraging the specialized capabilities of CROs in the realm of medical writing.
In October 2023, Medidata, a company under Dassault Systèmes and a prominent provider of clinical trial solutions in the life sciences sector, has announced the extension of its partnership with Catalyst Clinical Research over several years. The extended partnership between Medidata and Catalyst Clinical Research, with a focus on supporting Catalyst's global oncology brand, Catalyst Oncology, is poised to benefit medical writing services in several ways. The integration of additional offerings such as Medidata Grants Manager and Medidata AI Intelligent Trials enhances the capabilities of Catalyst as a full-service clinical research organization. These tools provide advanced functionalities, streamline processes, and offer intelligent solutions. For medical writing services, this means improved efficiency, access to comprehensive data, and the potential for more sophisticated analyses, ultimately contributing to the enhancement of documentation, regulatory submissions, and overall support for clinical trials in the field of oncology. Thus, such developments are fueling the studied market growth over the forecast period.
North America Segment is Expected to Witness Significant Growth Over the Forecast Period
North America stands as a significant hub for the medical writing industry, witnessing substantial growth and impact. The region's prominence in medical research, pharmaceuticals, and life sciences contributes to a robust demand for high-quality medical writing services. The presence of leading pharmaceutical companies, research institutions, and regulatory bodies in the United States and Canada further accentuates the need for effective medical writing to support clinical trials, regulatory submissions, and documentation.
In the United States, the pharmaceutical and biotechnology sectors have consistently driven innovation, leading to a surge in clinical research activities. Regulatory compliance and adherence to stringent documentation standards set by the U.S. Food and Drug Administration (FDA) make medical writing a critical component in the drug development process. Medical writing services play a vital role in preparing regulatory submissions, including Investigational New Drug (IND) applications, New Drug Applications (NDAs), and other documentation crucial for regulatory approvals.
Furthermore, the recent development in the region is expected to fuel the growth of the studied market. For instance, in March 2023, ClinChoice Medical Development is delighted to confirm the completion of its acquisition of CROMSOURCE S.r.l., a full-service contract research organization with ISO certification. The acquisition of CROMSOURCE S.r.l. by ClinChoice Medical Development is poised to have a positive impact on medical writing services. This strategic move enhances ClinChoice's capabilities and global presence in the contract research organization sector, providing a broader platform for medical writing expertise. The integration of CROMSOURCE's resources and experience into ClinChoice's portfolio is expected to bolster and optimize medical writing services, offering clients an expanded range of high-quality solutions in clinical research and development.
Moreover, the advanced healthcare infrastructure, research funding, and the region's leadership in biomedical research create a conducive environment for medical writing expertise. As the North American region continues to witness advancements in medical technologies, biopharmaceuticals, and clinical research, the demand for specialized medical writing services is expected to remain robust, solidifying North America's status as a key player in the global medical writing landscape.
Get Complete Analysis Of The Report - Download Free Sample PDF
The medical writing market displays a moderate level of competitiveness, primarily defined by the existence of a select group of key players within its operational framework. These key players play a pivotal role in shaping the market dynamics and influencing its overall trajectory. Despite the relatively limited number of major contributors, the market is dynamic, marked by evolving trends, advancements in medical research, and the growing demand for specialized writing services. The competitive landscape is further influenced by factors such as the development of new therapeutics, increasing R&D expenditure, and the emergence of contract research organizations offering outsourcing services in the medical writing domain. As the industry continues to evolve, collaboration, innovation, and adaptability remain crucial for sustained competitiveness among market participants. The market including few players are:
Recent Development:
1) In August 2023, Celegence unveiled CAPTIS Copilot, an innovative document automation and literature review solution designed for the life sciences sector. This enterprise cloud-based platform utilizes pre-trained large language models (LLM) and Reinforcement Learning from Human Feedback (RLHF) specifically tailored for the device and diagnostic industry. CAPTIS Copilot marks a significant leap forward in enabling device and in vitro diagnostic (IVD) manufacturers to enhance innovation. It empowers clinical, regulatory, and medical writing teams by providing them with the tools to be more strategic and productive with their time. This cloud-based solution is poised to elevate efficiency and effectiveness within the life sciences domain.
2) In July 2023, Catalyst Clinical Research, a prominent provider of specialized solutions for clinical development, revealed today the successful acquisition of Genpro Research, a global clinical research organization headquartered in Massachusetts, with team members situated in the US, India, and Ireland. Genpro, recognized as a next-generation services and technology partner for the pharmaceutical, biotechnology, and medical devices sector, specializes in areas such as biometrics, medical writing, real-world evidence (RWE), and AI-enabled automation product development. Dr. Sachin Marulkar and the current management team will continue to lead Genpro under the new ownership. The acquisition of Genpro Research by Catalyst Clinical Research is expected to positively impact the medical writing market by fostering growth and innovation within the sector. Genpro, being a next-generation services and technology partner with expertise in biometrics, medical writing, real-world evidence (RWE), and AI-enabled automation product development, brings valuable capabilities to Catalyst.
Q1. What was the Medical Writing Market size in 2023?
As per Data Library Research the global medical writing market size was valued at USD 4.1 billion in 2023.
Q2. What are the Growth Drivers of the Medical Writing Market?
Medical Writing Market is expected to expand at a CAGR of 10.46% over the forecast period.
Q3. Which Region is expected to hold the highest Market share?
North America region is expected to hold the highest Market share.
Q4. Who are the key players in Medical Writing Market?
Some key players operating in the market include
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
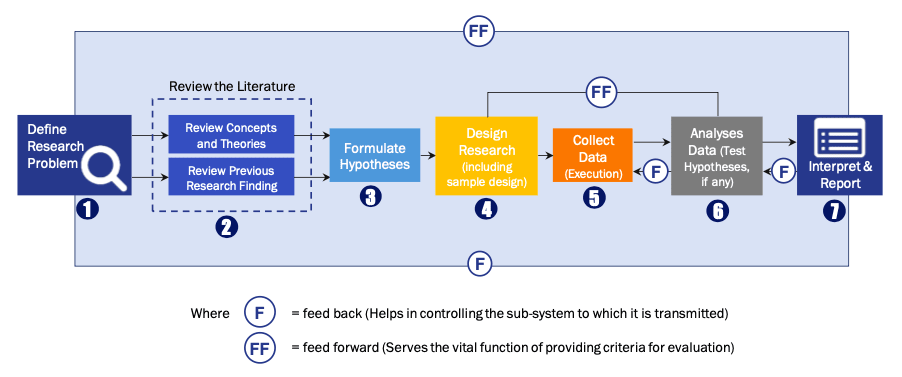
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model