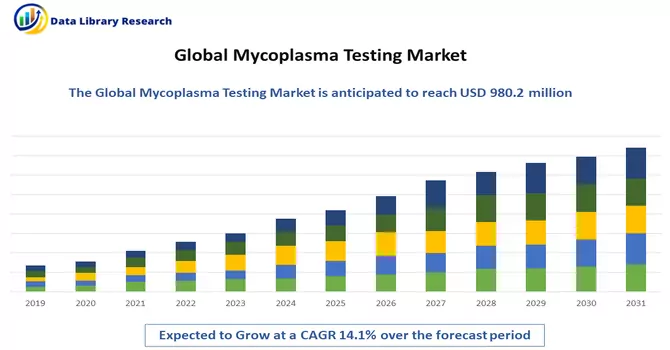
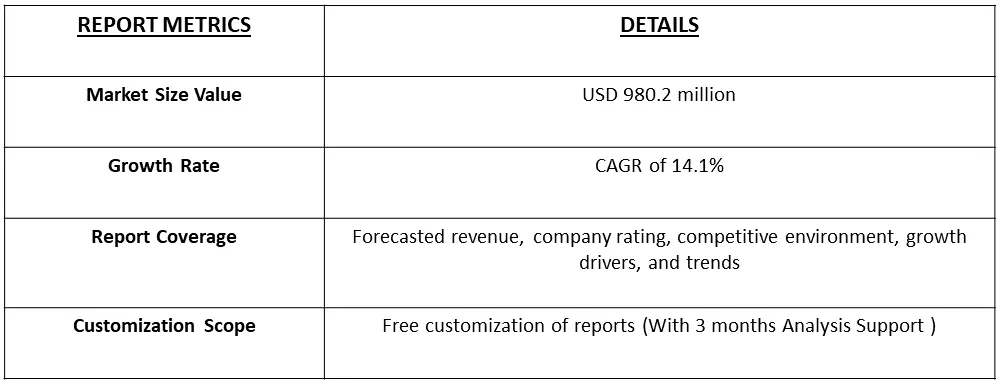
The global mycoplasma testing market size was estimated at USD 980.2 million in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 14.1% from 2024 to 2031.
Get Complete Analysis Of The Report - Download Free Sample PDF
Mycoplasma testing involves the detection of mycoplasma contamination in cell cultures, crucial for maintaining the integrity of cell-based research. Mycoplasma, bacteria lacking a cell wall, can contaminate cultures through various sources. Detection methods include microscopic examination, DNA staining, PCR, and culture-based techniques. Regular testing is essential to ensure reliable experimental results and prevent the spread of contamination between cell cultures, as mycoplasma contamination can compromise data accuracy and affect overall research outcomes. Researchers routinely employ these methods to identify and address mycoplasma contamination promptly.
The market is experiencing positive growth due to increasing investments in research and development (R&D). The demand for mycoplasma tests is being fueled by the widespread adoption of new technologies in drug research and development, coupled with advancements in cell culture technology. A notable example is SwiftDx, which introduced an innovative mycoplasma detection kit in February 2023. This newly launched SwiftDx Mycoplasma Detection Kit offers a more efficient solution for detecting mycoplasma contamination, utilizing a lateral flow test that ensures faster and more convenient results. This development underscores the industry's commitment to enhancing testing methodologies, contributing to the overall progress and efficiency of research and development endeavors.
The Mycoplasma testing market is characterized by several significant trends. Increasing R&D investments in biopharmaceutical and biotechnology sectors drive the demand for reliable testing methods, ensuring the integrity of cell cultures. Ongoing technological advancements, particularly in molecular biology and diagnostics, contribute to the adoption of innovative detection methods such as PCR-based assays and rapid lateral flow tests. Key market players are engaged in strategic collaborations and mergers, expanding their market presence and fostering the development of integrated solutions. The focus on point-of-care testing is growing, with user-friendly, on-site testing kits gaining popularity for their speed and convenience. Additionally, the market is witnessing an expansion of applications in cell culture technology across various industries. Furthermore, there is a notable trend of incorporating Mycoplasma testing within panels targeting sexually transmitted infections, addressing the broader healthcare diagnostic market with comprehensive testing solutions. These trends collectively reflect the dynamic nature of the Mycoplasma testing market, shaped by technological innovations, strategic collaborations, and the evolving landscape of biopharmaceutical and biotechnology research.
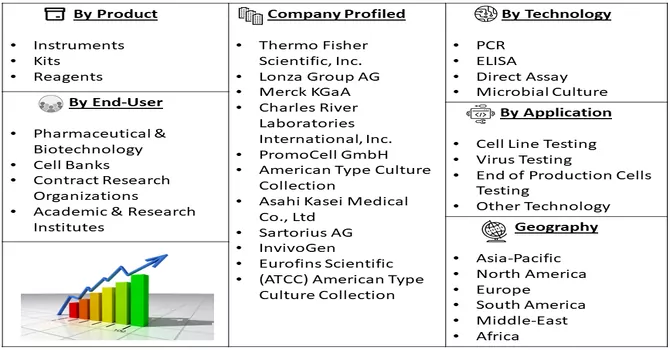
Market Segmentation: The Mycoplasma Testing Market is Segmented By Product (Instruments, Kits & Reagents), Technology (PCR, ELISA, Direct Assay, Microbial Culture), Application ( Cell Line Testing, Virus Testing, End of Production Cells Testing and Other Technology), End-use (Pharmaceutical & Biotechnology, Cell Banks, Contract Research Organizations, Academic & Research Institutes) and by geography (North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa). The report offers market size and forecasts for the global credit card market in value (USD billion) for all the above segments.
For Detailed Market Segmentation - Download Free Sample PDF
Market Drivers:
Increasing R&D investments in life sciences
The field of mycoplasma testing is experiencing a notable impact from the increasing research and development (R&D) investments within the broader life sciences sector. As life science researchers intensify their focus on advancing biopharmaceuticals, biotechnology, and related disciplines, the demand for robust mycoplasma testing methodologies is on the rise. The surge in R&D investments is driven by a collective effort to enhance drug development and therapeutic innovations, necessitating stringent quality control measures in cell cultures. This trend underscores the critical importance of reliable mycoplasma testing in maintaining the integrity of experimental data, ensuring the validity of research outcomes, and ultimately contributing to the advancement of cutting-edge therapies and medical solutions. As a result, the intersection of increasing R&D investments in life sciences and the imperative for accurate mycoplasma testing showcases a symbiotic relationship, where advancements in one area significantly contribute to the progress and reliability of the other.
Rising government support toward pharmaceutical and biotechnology industries
The mycoplasma testing market is experiencing positive momentum fueled by the rising support from government entities towards the pharmaceutical and biotechnology industries. Governments worldwide are increasingly recognizing the strategic importance of pharmaceutical and biotechnology sectors in advancing healthcare and fostering economic growth. This recognition translates into increased funding, grants, and supportive policies aimed at boosting research and development activities within these industries. As a crucial component of quality control in biopharmaceutical and biotechnological processes, mycoplasma testing is directly benefiting from this surge in government support. Government initiatives often include funding programs for research projects, infrastructure development, and collaborative efforts between research institutions and industry players. These initiatives aim to accelerate advancements in drug development, vaccine production, and other biotechnological innovations. Mycoplasma testing, being integral to maintaining the quality and safety of cell cultures, aligns with the regulatory standards enforced by these supportive government bodies. Moreover, the emphasis on public health and safety further amplifies the significance of mycoplasma testing in pharmaceutical and biotechnology applications. Governments recognize the role of stringent testing protocols, including mycoplasma testing, in ensuring the efficacy and safety of biopharmaceutical products. In essence, the rising government support toward the pharmaceutical and biotechnology industries serves as a catalyst for the growth of the mycoplasma testing market. It not only provides financial backing for research activities but also fosters an environment conducive to innovation, collaboration, and the development of advanced testing methodologies within the broader life sciences landscape. This symbiotic relationship between government support and the mycoplasma testing market underscores the interconnectedness of regulatory frameworks, industry growth, and the pursuit of advancements in healthcare.
Market Restraints:
High cost of research in cell biology
The Mycoplasma Testing Market faces the potential challenge of internal factors that may slow its own growth. One such factor is the market saturation that could result from an increased number of players and products entering the arena. As the market becomes more crowded, competition intensifies, potentially leading to pricing pressures and a fragmented landscape. Additionally, redundancy in product offerings may emerge, diluting the uniqueness of individual solutions. Furthermore, the market may face a hindrance in growth due to the time-consuming nature of regulatory approvals for new mycoplasma testing methodologies or products. Stringent regulatory processes, though essential for ensuring the safety and efficacy of testing methods, can significantly extend the time-to-market for new innovations, impacting the speed at which the market can evolve and expand. The adoption of alternative testing methods or technologies may also pose a challenge to the Mycoplasma Testing Market. If alternative approaches gain prominence and prove to be more cost-effective or efficient, they could divert attention and resources away from traditional mycoplasma testing methods. Moreover, economic uncertainties and budget constraints within research institutions and biopharmaceutical companies may lead to a cautious approach in investing in advanced mycoplasma testing solutions. In times of financial constraints, organizations might prioritize essential expenditures over adopting newer, potentially more expensive testing technologies. In navigating these challenges, industry players will need to focus on continuous innovation, differentiation, and strategic collaborations to overcome potential market slowdowns. Proactive efforts to address regulatory hurdles, streamline approval processes, and emphasize the unique benefits of mycoplasma testing solutions will be crucial in sustaining growth despite these internal challenges.
The COVID-19 pandemic has significantly impacted the Mycoplasma Testing Market, both directly and indirectly. On one hand, the heightened focus on healthcare and biosafety during the pandemic has underscored the importance of stringent quality control measures, including mycoplasma testing, in the development and production of pharmaceuticals and biotechnology products. The need to ensure the integrity of cell cultures and research outcomes has become even more critical, amplifying the demand for reliable mycoplasma testing solutions. However, the pandemic has also brought about challenges for the Mycoplasma Testing Market. Disruptions in the supply chain, travel restrictions, and temporary closures of laboratories during lockdowns have led to delays in research activities and hindered the procurement of testing supplies. This has impacted the pace of mycoplasma testing initiatives and research projects, particularly in regions heavily affected by the pandemic. Additionally, the redirection of resources and attention towards COVID-19-related research has potentially diverted focus and funding away from other areas, including mycoplasma testing. Laboratories and pharmaceutical companies may have reprioritized their budgets and research efforts to address the immediate challenges posed by the pandemic, affecting the growth trajectory of the mycoplasma testing sector. The adoption of remote work practices and virtual collaboration tools has become more prevalent during the pandemic, influencing how mycoplasma testing services are conducted and accessed. Virtual collaborations and remote monitoring tools have become increasingly important in ensuring the continuity of research and testing activities. Looking ahead, as the world transitions into a post-pandemic era, the Mycoplasma Testing Market is expected to rebound, driven by the continued emphasis on biosafety, the resumption of delayed research projects, and the evolving landscape of healthcare priorities. The lessons learned during the pandemic regarding the importance of robust testing and biosafety measures are likely to contribute to the long-term growth and resilience of the mycoplasma testing industry.
Segmental Analysis:
Kits & Reagents Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
The Mycoplasma Testing Market is significantly influenced by the segment of kits and reagents, which plays a pivotal role in ensuring the accuracy and efficiency of mycoplasma testing methodologies. Kits and reagents are essential components that provide researchers, laboratories, and biopharmaceutical companies with the necessary tools to conduct robust and reliable mycoplasma testing. The demand for mycoplasma testing kits and reagents is fueled by the increasing need for streamlined, user-friendly testing solutions. These kits typically include essential components such as PCR reagents, DNA staining dyes, and other specialized materials designed to detect and identify mycoplasma contamination in cell cultures. The convenience and efficiency offered by these kits contribute to their widespread adoption in research laboratories and biomanufacturing facilities. Moreover, the continuous advancements in technology and the development of innovative testing methodologies are reflected in the evolution of mycoplasma testing kits. Rapid lateral flow assays, for instance, have gained prominence for their quick and convenient detection capabilities, further boosting the market for mycoplasma testing kits and reagents. The affordability and accessibility of these kits make them crucial for a diverse range of applications, from academic research to industrial-scale biopharmaceutical production. The availability of ready-to-use kits simplifies the mycoplasma testing process, reducing the complexities associated with in-house assay development and enhancing the overall efficiency of testing procedures. As the biopharmaceutical and biotechnology industries continue to expand, the kits and reagents segment of the Mycoplasma Testing Market is expected to witness sustained growth. Industry players are likely to focus on developing advanced, cost-effective kits that cater to the evolving needs of researchers and manufacturing facilities. This segment's dynamic nature emphasizes its integral role in supporting the overall growth and reliability of mycoplasma testing methodologies in diverse scientific and industrial settings.
ELISA Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
Enzyme-Linked Immunosorbent Assay (ELISA) plays a significant role in the Mycoplasma Testing Market, offering a versatile and widely adopted method for detecting and quantifying mycoplasma contamination. ELISA is an immunological assay that utilizes the binding specificity of antibodies to detect the presence of mycoplasma antigens in cell cultures or other biological samples. The ELISA technique provides a sensitive, high-throughput, and cost-effective solution for mycoplasma testing, making it a popular choice in research laboratories and biopharmaceutical manufacturing. ELISA-based mycoplasma testing involves the immobilization of mycoplasma antigens on a solid surface, followed by the binding of specific antibodies that are conjugated with enzymes. The subsequent enzymatic reaction produces a measurable color change, indicating the presence of mycoplasma contamination. This method allows for the rapid screening of large numbers of samples, making it particularly valuable in quality control processes within the biopharmaceutical industry. The ELISA-based approach provides advantages such as sensitivity, specificity, and the ability to quantify mycoplasma levels. Its adaptability to various sample types and compatibility with automation further contribute to its widespread adoption. ELISA kits designed specifically for mycoplasma testing are commercially available, offering researchers and industry professionals standardized and convenient solutions for detecting and preventing mycoplasma contamination in cell cultures. As the demand for reliable and efficient mycoplasma testing continues to rise, ELISA technology is expected to play a pivotal role in meeting these requirements. Ongoing advancements in ELISA methodologies, including improvements in sensitivity and automation, further contribute to the growth and versatility of ELISA within the dynamic landscape of the Mycoplasma Testing Market.
Cell Line Testing Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
Cell Line Testing is a critical component of the Mycoplasma Testing Market, integral to ensuring the integrity and reliability of cell cultures utilized in various research, biopharmaceutical, and biotechnology applications. Mycoplasma contamination poses a significant threat to cell lines, potentially compromising experimental outcomes and the quality of biopharmaceutical products. Cell line testing, with a specific focus on mycoplasma, involves the systematic screening and monitoring of cell cultures to detect the presence of these bacteria. The Cell Line Testing segment in the Mycoplasma Testing Market encompasses various methodologies, including PCR-based assays, enzyme-linked immunosorbent assays (ELISA), and traditional culture methods. PCR-based techniques offer high sensitivity and specificity, enabling rapid and accurate identification of mycoplasma DNA. ELISA provides an immunological approach, detecting mycoplasma antigens, while culture-based methods involve growing cells on specific media to observe mycoplasma contamination visually. The importance of comprehensive cell line testing, including mycoplasma testing, is underscored by regulatory standards and guidelines governing the development and production of biopharmaceuticals. Quality control measures mandate rigorous testing protocols to ensure the absence of mycoplasma contamination, aligning with Good Cell Culture Practice (GCCP) and other industry-specific regulations.The Cell Line Testing market is driven by the increasing adoption of cell culture technology in research and manufacturing processes, necessitating reliable mycoplasma testing solutions. Biopharmaceutical companies, academic institutions, and research laboratories rely on these tests to maintain the authenticity of cell lines, supporting the production of safe and effective therapeutic products. In summary, the Cell Line Testing segment within the Mycoplasma Testing Market is a critical component in safeguarding the quality and reliability of cell cultures. As the demand for advanced and efficient mycoplasma testing solutions continues to rise, this segment plays a pivotal role in contributing to the overall growth and success of the Mycoplasma Testing Market.
Pharmaceutical & Biotechnology Companies Segment is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
Pharmaceutical and biotechnology companies are pivotal players in driving the growth of the Mycoplasma Testing Market, as they heavily rely on stringent quality control measures to ensure the safety and efficacy of their products. Mycoplasma contamination poses a significant risk to cell cultures, impacting the reliability of experimental data and the production of biopharmaceuticals. Consequently, the implementation of robust mycoplasma testing protocols is paramount within these industries. Quality control in pharmaceutical and biotechnology companies involves routine screening and monitoring of cell cultures to detect mycoplasma contamination. The Mycoplasma Testing Market caters to the diverse needs of these companies by offering a range of testing methodologies, including PCR-based assays, culture-based methods, and rapid lateral flow tests. These companies prioritize accurate and efficient mycoplasma testing solutions to safeguard their research, development, and manufacturing processes. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), mandate stringent guidelines for the biopharmaceutical industry, emphasizing the necessity of mycoplasma testing in ensuring product safety and compliance. Pharmaceutical and biotechnology companies integrate these testing procedures into their quality assurance programs to adhere to regulatory standards and mitigate the risk of mycoplasma contamination.
The Mycoplasma Testing Market caters to the evolving needs of pharmaceutical and biotechnology companies by offering innovative testing kits, reagents, and services that align with industry best practices. As the demand for biopharmaceutical products continues to rise, driven by advancements in therapeutic research and development, the importance of mycoplasma testing in maintaining the integrity of cell cultures becomes even more critical. Moreover, pharmaceutical and biotechnology companies are actively engaged in collaborations with mycoplasma testing solution providers, contributing to the market's growth through joint research efforts and the development of cutting-edge testing technologies. Overall, the symbiotic relationship between pharmaceutical and biotechnology companies and the Mycoplasma Testing Market underscores the significance of stringent quality control measures in ensuring the success of innovative research and the production of safe and effective biopharmaceutical products.
North America Region is Expected to Witness is Expected to Witness Significant Growth Over the Forecast Period
North America emerged as the dominant region in the market, capturing a substantial share, as the region's leading position can be attributed to several factors, including a heightened prevalence of respiratory disorders and substantial investments by major biotechnology companies. These investments are particularly driven by the increasing adoption of biotechnology in cancer research and the development of novel biologics, vaccines, and pharmaceuticals. Additionally, key organizations such as the FDA and the U.S. Department of Health and Human Services collaborate with the Centers for Disease Control and Prevention and the National Institutes of Health. Their concerted efforts focus on enhancing standards for biological and microbiological safety testing in biomedical laboratories, evident through the formulation and enforcement of rigorous regulatory guidelines. This proactive approach contributes to North America's significant market share by ensuring a robust framework for maintaining safety and quality standards within the biotechnology and biomedical research sectors.
Get Complete Analysis Of The Report - Download Free Sample PDF
Prominent entities in this market are strategically employing diverse tactics, such as engaging in partnerships via mergers and acquisitions, expanding geographically, and fostering strategic collaborations. These initiatives aim to strengthen and broaden their market presence. By undertaking these strategic measures, key players are positioning themselves to capitalize on emerging opportunities, enhance their competitive standing, and foster sustainable growth within the market. This dynamic landscape underscores the industry's commitment to proactive and versatile strategies, fostering a competitive and innovative environment. Key Mycoplasma Testing Companies:
Recent Development:
1) In April 2023, Agathos Biologics unveiled its analytical testing services catering to life science researchers. Notably, Agathos relies exclusively on QIAcuity instrumentation and assays developed by QIAGEN for its testing services. Additionally, a strategic collaboration has been forged between Agathos and QIAGEN, focusing on testing and validation for mycoplasma and recombinant adeno-associated viral vector (rAAV) assays. This collaboration underscores Agathos Biologics' commitment to leveraging advanced technologies and partnerships to enhance its analytical testing capabilities in the life sciences domain.
2) In February 2023, Thermo Fisher Scientific, Inc. introduced the TrueMark STI Select Panel, a significant development in the detection of sexually transmitted infectious pathogens. This innovative panel is designed to identify the four most common pathogens using polymerase chain reaction technology, specifically detecting Chlamydia trachomatis and Mycoplasma genitalium. Thermo Fisher Scientific's launch of the TrueMark STI Select Panel demonstrates its dedication to providing comprehensive and efficient diagnostic solutions for sexually transmitted infections, addressing a critical need in healthcare and emphasizing the company's commitment to advancing diagnostic capabilities.
Q1. What is the current Mycoplasma Testing Market size?
The global mycoplasma testing market size was estimated at USD 980.2 million in 2023.
Q2. What is the Growth Rate of the Mycoplasma Testing Market?
Mycoplasma Testing Market is expected to grow at a compound annual growth rate (CAGR) of 14.1% over the forecast period.
Q3. Which Region is expected to hold the highest Market share?
North America region is expected to hold the highest Market share.
Q4. What are the factors on which the Mycoplasma Testing Market research is based on?
By Product, By Technology, By Application, End User & Geography are the factors on which the Mycoplasma Testing Market research is based.
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS
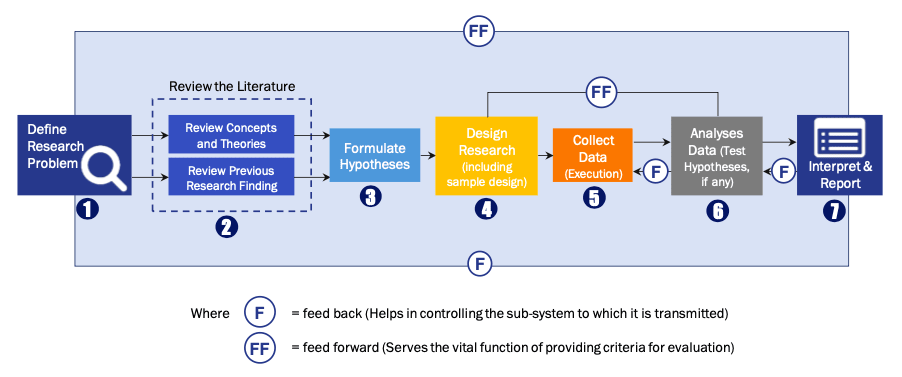
Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model