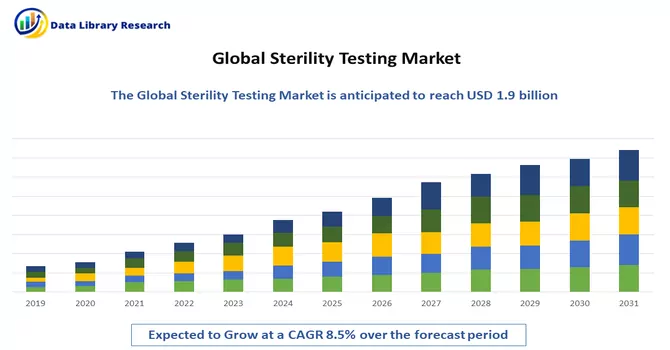
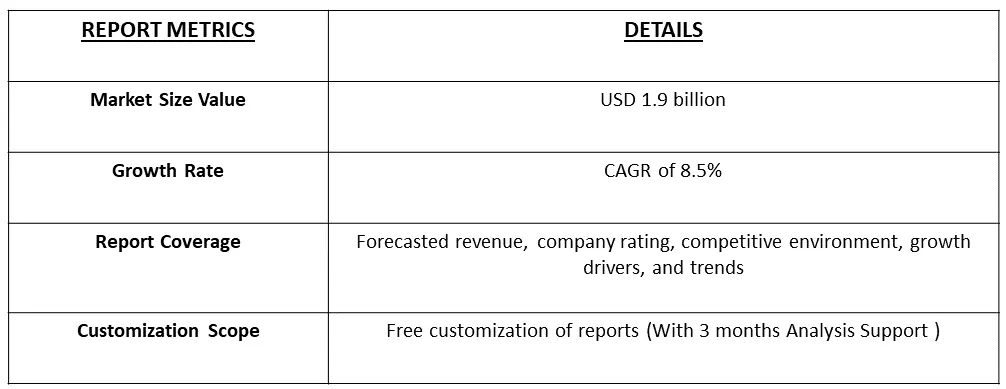
The global sterility testing market is anticipated to reach a valuation of USD 1.9 billion by 2023, with a projected Compound Annual Growth Rate (CAGR) of 8.5% from 2023 to 2030.
Get Complete Analysis Of The Report - Download Free Sample PDF
This market segment is integral to the examination of pharmaceutical and biotechnology products, ensuring their freedom from viable microorganisms. Sterility testing plays a pivotal role in the manufacturing process of sterile pharmaceuticals, serving as a vital step in confirming the absence of harmful microorganisms that could compromise product safety and efficacy.
The industry is heavily influenced by stringent regulations and guidelines established by prominent health authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations mandate rigorous sterility testing procedures to guarantee the quality and safety of pharmaceutical products. Consequently, the regulatory environment acts as a significant driver for the demand in sterility testing services. The escalating prevalence of chronic diseases and the surging demand for injectable drugs, biologics, and other sterile pharmaceuticals contribute to the growing need for effective sterility testing. In the healthcare industry, where patient safety and infection prevention are paramount concerns, the importance of robust sterility testing is underscored. As a result, the market continues to experience sustained growth driven by these critical factors.
There's a notable trend in the industry towards embracing rapid sterility testing methods, which prioritize delivering faster results without compromising on the essential aspects of sensitivity and accuracy. Cutting-edge technologies like molecular methods and automated systems are becoming increasingly prominent due to their potential to significantly reduce testing durations. Moreover, there's a continued emphasis on advancing and incorporating sophisticated analytical technologies such as next-generation sequencing (NGS) and polymerase chain reaction (PCR) for sterility testing applications. These innovative technologies bring about heightened levels of sensitivity and specificity, enabling a more precise and reliable detection of microorganisms.
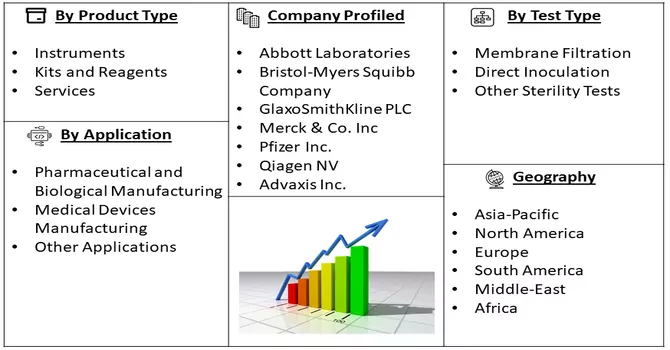
Market Segmentation: The Sterility Testing Market is Segmented by Product Type (Instruments, Kits and Reagents, and Services), Test Type (Membrane Filtration, Direct Inoculation, and Other Sterility Tests), Application (Pharmaceutical and Biological Manufacturing, Medical Devices Manufacturing, and Other Applications), and Geography (North America, Europe, Asia-Pacific, Middle-East and Africa, and South America). The report offers the value (in USD million) for the above segments.
For Detailed Market Segmentation - Download Free Sample PDF
Market Drivers:
Increasing Number of Chronic Diseases
The escalating prevalence of chronic diseases is anticipated to drive the demand for effective drugs, consequently fueling the need for comprehensive sterility testing of pharmaceuticals prior to their market introduction. This surge in demand is exemplified by recent developments, such as the Government of India's September 2022 launch of a vaccine against cervical cancer, the Quadrivalent Human Papillomavirus vaccine (qHPV), developed by the Serum Institute of India (SII) and the Department of Biotechnology (DBT). Additionally, in the same month, Bayer introduced vericiguat in India to mitigate the risk of cardiovascular deaths and recurrent hospitalizations. These instances underscore the critical role of sterility testing in ensuring the safety and efficacy of drugs, aligning with the broader market growth trajectory.
Increasing Demand for Biopharmaceuticals and Sterile Products:
The increasing need for biopharmaceuticals and sterile pharmaceutical products stands out as a key factor propelling the sterility testing market. The pharmaceutical industry is witnessing a rising demand for biologics, gene therapies, and injectable drugs, driven by their effectiveness in addressing diverse medical conditions. Manufacturing these products necessitates a stringent focus on sterility assurance to ward off contamination and uphold patient safety. With the continuous expansion of the biopharmaceutical sector, there is a parallel surge in the requirement for robust sterility testing methodologies, essential for preserving the quality and safety standards of these advanced therapeutic products.
Market Restraints:
Stringent Regulatory Framework
The anticipated impediment to the growth of the sterility testing market in the forecast period lies in the formidable regulatory framework. The stringent regulations set forth by health authorities, including but not limited to the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are poised to act as a restraining force. Compliance with these rigorous regulatory standards is imperative for ensuring the safety and efficacy of pharmaceutical and biotechnology products, thereby becoming a pivotal factor in the market dynamics. As the industry navigates through this demanding regulatory landscape, it is expected to encounter challenges that might slow down the overall growth trajectory of the sterility testing market. Meeting and sustaining compliance with evolving regulatory requirements is likely to necessitate considerable investments and resources, thereby potentially hindering the pace of market expansion over the forecast period.
The pandemic has highlighted the importance of healthcare preparedness and the need for rapid, accurate, and widespread testing. The trajectory of the sterility testing market was significantly influenced by the COVID-19 pandemic. Sterility testing emerged as a crucial tool for numerous pharmaceutical and biotechnological companies as they redirected their focus toward research and development, particularly in identifying leads for diagnosing and treating SARS-CoV-2 infections. The surge in COVID-19 cases prompted major companies and research institutions to explore unconventional medical approaches, resulting in substantial investments in research and development efforts globally by both private and public entities. These endeavors have had a profound positive impact on the sterility testing market. The heightened severity and rapid spread of infections, coupled with the escalating development of drugs and vaccines, led to an increased demand for sterility testing in the pharmaceutical and biopharmaceutical production sectors. This, in turn, heightened the necessity for rigorous sterility testing protocols for products. The growing adoption and demand for sterility testing indicate a positive trend, and the market is anticipated to regain its full potential over the next two to three years.
Segmental Analysis:
Instruments, Kits and Reagents Segment is Expected to Witness Significant Growth Over the Forecast Period
The sterility testing instruments include a range of equipment and devices used to perform tests that detect the presence of viable microorganisms in pharmaceutical products. These instruments are designed to meet the specific requirements of sterility testing protocols. Moreover, microbial air samplers, membrane filtration systems, automated incubation systems, real-time PCR instruments, and other microbiological testing equipment. Sterility testing kits consist of pre-packaged sets of materials and consumables required to perform specific sterility tests. These kits often include sterile containers, filtration membranes, growth media, and other components necessary for the testing process. Also, membrane filtration kits, closed sterility test systems, and other packaged kits designed for specific sterility testing applications. Sterility testing reagents are substances or compounds used in the testing process to facilitate the detection and identification of microorganisms. These can include growth media, staining reagents, and other chemical solutions. Culture media (e.g., nutrient agar), microbial identification reagents, and dyes used in staining procedures. Thus, owing to such advantages the segment is expected to witness significant growth over the forecast period.
Membrane Filtration Segment is Expected to Witness Significant Growth Over the Forecast Period
The basic principle of membrane filtration involves passing a liquid sample through a sterile membrane filter. The membrane acts as a physical barrier, trapping microorganisms present in the sample. The membrane is then placed on an appropriate culture medium, and any microbial growth is observed and analyzed. Membrane filtration is commonly used in sterility testing for a variety of pharmaceutical products, including injectable drugs, parenteral solutions, and ophthalmic preparations. It is also employed in testing raw materials, environmental monitoring, and testing of medical devices. Thus, owing to such advantages the segment is expected to witness significant growth over the forecast period.
North America Region is Expected to Witness Significant Growth Over the Forecast Period
North America, particularly the United States, is home to a robust pharmaceutical and biotechnology industry. The presence of major pharmaceutical companies and a thriving biotech sector contributes significantly to the demand for sterility testing services. The regulatory environment in North America, governed by agencies such as the U.S. Food and Drug Administration (FDA) and Health Canada, imposes stringent standards on the quality and safety of pharmaceutical products. Sterility testing is a critical component of regulatory compliance, driving the demand for reliable testing services and products.
Additionally, the growth of the market is being driven by an upsurge in drug launches and an increasing corporate focus on conducting diverse clinical trials within the region to address various chronic, infectious, and other diseases. For example, in March 2022, VBI Vaccines Inc. introduced PreHevbrioin in the United States. This vaccine aims to prevent infections caused by all known subtypes of the hepatitis B virus (HBV) in adults aged 18 years and older. Notably, the FDA approved this vaccine as the sole 3-antigen HBV vaccine for adults in November 2021. The mounting incidence of diabetes, coupled with heightened investments from the Canadian government in diabetes research and development, is a significant driver of market growth. In a noteworthy move, Canada's Budget 2021 proposed new investments in diabetes research, surveillance, prevention, and the establishment of a national framework for diabetes. As part of this initiative, the government of Canada, through the Canadian Institutes of Health Research (CIHR), expressed its commitment to the JDRF-CIHR partnership to Defeat Diabetes. The government announced an investment of up to USD 15 million, with a total research impact estimated at USD 30 million. These government initiatives are expected to be key contributors to market growth over the forecast period.
Thus, above-mentioned factors such as substantial R&D spending, government investments, and the ongoing influx of new drug launches position the studied market for growth in the foreseeable future.
Get Complete Analysis Of The Report - Download Free Sample PDF
The competitive landscape of the sterility testing market is characterized by moderate competition, featuring a number of key players. As the biopharmaceutical sector continues to present lucrative opportunities, there is an anticipation of new entrants into the market. Among the prominent players in this landscape are:
Recent Developments:
1) In June 2022, STEMart introduced an extensive microbiology and sterility testing service tailored for sterile, non-pyrogenic medical devices.
2) In June 2022, Berkshire Sterile Manufacturing inaugurated a sterility testing isolator designed for conducting onsite sterility testing for its GMP (Good Manufacturing Practice) batches. The company specializes in the sterile filling of injectable medicines, primarily those involved in clinical trials or with a limited commercial demand.
Q1. What was the Sterility Testing Market size in 2023?
As per Data Library Research Market the global sterility testing market is anticipated to reach a valuation of USD 1.9 billion by 2023.
Q2. What is the Growth Rate of the Sterility Testing Market?
Sterility Testing Market a projected Compound Annual Growth Rate (CAGR) of 8.5% over the forecast period.
Q3. What segments are covered in the Sterility Testing Market Report?
By Product Type, Test Type, By Application, End-User and Geography these segments are covered in the Sterility Testing Market Report.
Q4. What are the factors driving the Sterility Testing Market?
Key factors that are driving the growth include the Increasing Number of Chronic Diseases and Increasing Demand for Biopharmaceuticals and Sterile Products
Data Library Research are conducted by industry experts who offer insight on industry structure, market segmentations technology assessment and competitive landscape (CL), and penetration, as well as on emerging trends. Their analysis is based on primary interviews (~ 80%) and secondary research (~ 20%) as well as years of professional expertise in their respective industries. Adding to this, by analysing historical trends and current market positions, our analysts predict where the market will be headed for the next five years. Furthermore, the varying trends of segment & categories geographically presented are also studied and the estimated based on the primary & secondary research.
In this particular report from the supply side Data Library Research has conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and SOFT) of the companies that active & prominent as well as the midsized organization
FIGURE 1: DLR RESEARH PROCESS

Extensive primary research was conducted to gain a deeper insight of the market and industry performance. The analysis is based on both primary and secondary research as well as years of professional expertise in the respective industries.
In addition to analysing current and historical trends, our analysts predict where the market is headed over the next five years.
It varies by segment for these categories geographically presented in the list of market tables. Speaking about this particular report we have conducted primary surveys (interviews) with the key level executives (VP, CEO’s, Marketing Director, Business Development Manager and many more) of the major players active in the market.
Secondary ResearchSecondary research was mainly used to collect and identify information useful for the extensive, technical, market-oriented, and Friend’s study of the Global Extra Neutral Alcohol. It was also used to obtain key information about major players, market classification and segmentation according to the industry trends, geographical markets, and developments related to the market and technology perspectives. For this study, analysts have gathered information from various credible sources, such as annual reports, sec filings, journals, white papers, SOFT presentations, and company web sites.
Market Size EstimationBoth, top-down and bottom-up approaches were used to estimate and validate the size of the Global market and to estimate the size of various other dependent submarkets in the overall Extra Neutral Alcohol. The key players in the market were identified through secondary research and their market contributions in the respective geographies were determined through primary and secondary research.
Forecast Model
